Seeing Systemic Disease within the EyeThis month in Review of Optometry, multiple ODs explain the connection between the eye and various systemic conditions, including hypertension, stroke, sleep disorders, COVID-19, diabetes, thyroid eye disease and more. Check out the other articles featured in the October issue:
|
Hypertension affects 47.3% of the adult United States population, with one in five of these individuals unaware of their condition and only one in four having it under control.1 Meanwhile, one stroke occurs every 40 seconds and one death from stroke every 3.5 minutes in the US.2 It is no coincidence that hypertension is the leading cause of stroke.3
Given the prevalence of these conditions, optometrists in a primary care setting encounter affected patients daily. When primary eyecare providers examine the blood vessels of the eyes, it yields clues to systemic vascular status. As a result, it is important to address and manage the ocular manifestations of hypertension and stroke, which could potentially save lives.
First and foremost, the patient’s symptoms on an initial intake or history form may elevate level of suspicion. Risk factors for hypertension and stroke that can be gathered from the history form alone include the following:3
- Age >65 years old
- African-American race
- Male sex
- Obesity
- Tobacco/alcohol consumption
- (+) Family history
- Comorbidities:
- Diabetes
- Cardiovascular disease
- High cholesterol
- Sleep apnea
- Kidney disease
 |
|
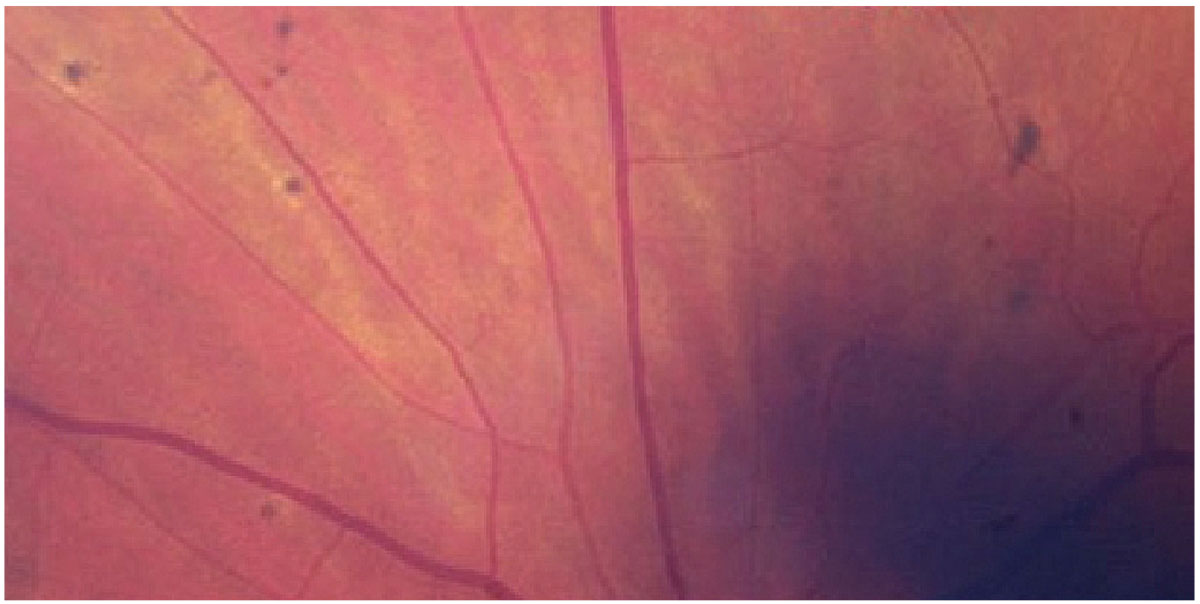
Fig. 1. Elschnig spots seen in a patient with hypertensive retinopathy. Click image to enlarge. |
Hypertension on Routine Exam
In addition to taking a thorough history, make blood pressure (BP) measurement a part of the routine exam for patients with the above-mentioned risk factors. Not only is this the best way to assess for control in existing hypertensive patients, but it can also alert them about undiagnosed hypertension, as the condition is largely asymptomatic in nature.
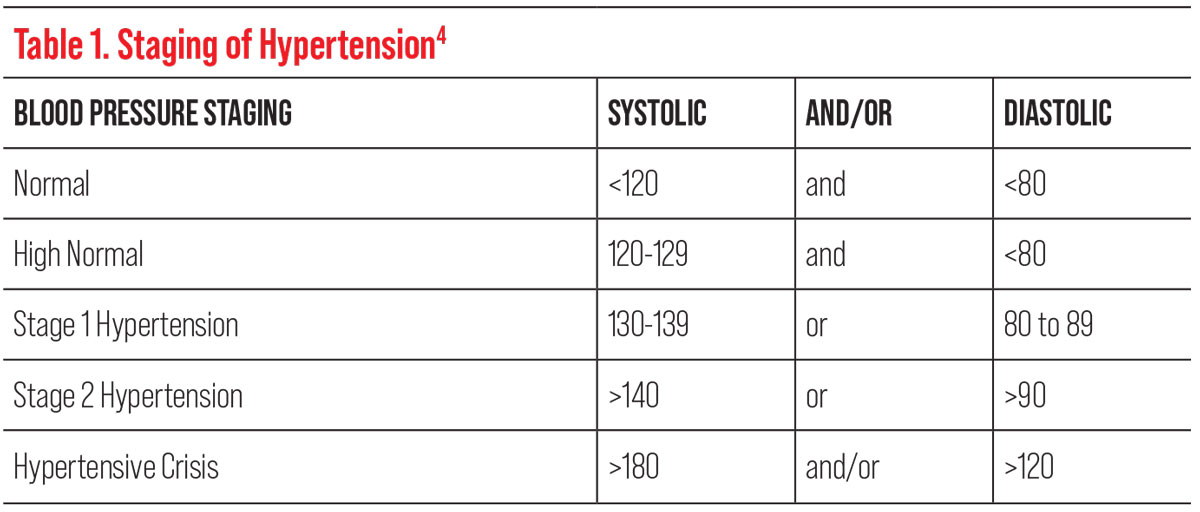 |
| Table 1. Click image to enlarge. |
The diagnosis of hypertension is made through serial BP readings taken throughout the day, so having more information may aid the patient or their primary care provider in the diagnosis. In 2017, the American College of Cardiology and American Heart Association (AHA) lowered the threshold blood pressure reading for the diagnosis of hypertension to ≥130/80, as studies found that doing so reduced the risk of mortality by 27%.4
In patients with elevated BP readings in-office (the aforementioned ≥130/80), it is important to assess for headaches, arrhythmia, vision loss, tinnitus, fatigue, nausea, vomiting, confusion, chest pain, tremors, muscle weakness and tingling or numbness, as these are all common signs and symptoms of a stroke requiring urgent evaluation.2 If a patient presents with hypertensive crisis (>180/120), even without symptoms, at the least an attempt should be made to call the general physician; with no other alternative, the patient should be advised to seek immediate care at an emergency room.
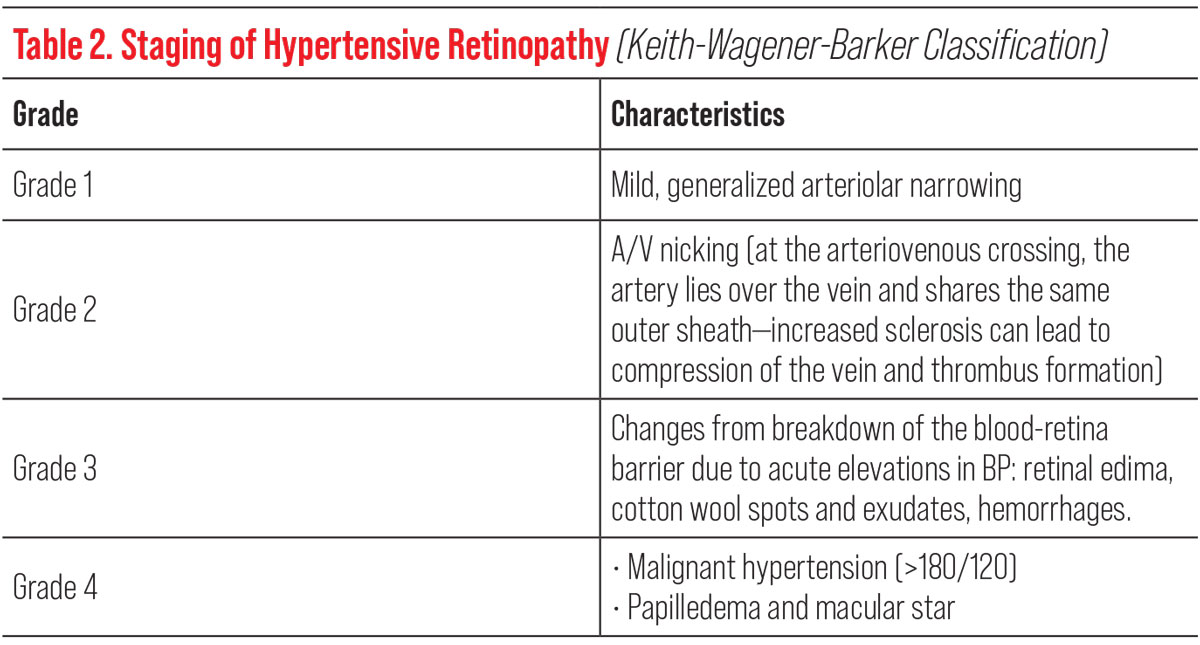 |
| Table 2. Click image to enlarge. |
In asymptomatic patients with stage 1 or stage 2 hypertension, dilation without phenylephrine is indicated due to the drop’s potential to elevate blood pressure as a vasoconstrictor (Table 1). If phenylephrine is required to gain access to the retinal periphery, punctal occlusion can be used to prevent systemic absorption.
The pathophysiology of benign essential hypertension, which accounts for 95% of hypertensive patients, is complex.1,3 No single identifiable cause accounts for elevated BP in these patients but rather a combination of lifestyle and genetic risk factors. The other 5% of hypertensive patients have a direct, underlying renal or adrenal disease.1,3 BP elevates when normal cardiac output is met by increased peripheral resistance in the arterioles due to vasoconstriction. The main culprit behind excess vasoconstriction is an overactive sympathetic nervous system.5 This causes endothelial cell dysfunction in medium- to large-sized arteries, which leads to thrombus formation and turbulent blood flow, and ultimately increases the risk for clot formation.5
The inner retinal vasculature is autoregulated—meaning that there is an absence of sympathetic nerve supply, unlike the choroidal vasculature that only has a sympathetic nerve supply.6 Elevated BP in the background of autoregulation initially causes vasoconstriction, but persistent elevation overrides this compensatory mechanism, leading to vasospasms, thickening of the vessel layers and endothelial damage.7 As a result, chronic hypertension leads to retinal arteriolar narrowing and, eventually, the breakdown of the blood-retinal barrier.
These changes can be observed funduscopically as increased arterial light reflexes, artery-vein compression, cotton wools spots or inner retinal hemorrhages with exudates.7,8 Meanwhile, hypertensive choroidopathy will present as areas of focal infarcts, seen funduscopically as Siegrist streaks and Elschnig spots.9 Although these are changes that may be seen on routine exam in hypertensive patients, the presence of grade 2 hypertensive retinopathy and beyond increases the long-term risk for stroke and suggests the need for tighter BP control (Table 2).10
Keep an Eye Out
Transient monocular vision loss (TMVL), transient ischemic attacks (TIA), branch retinal artery occlusions (BRAO) and central retinal artery occlusions (CRAO) are all forms of acute retinal arterial ischemia that are considered ocular and systemic emergencies requiring proper and immediate diagnosis and management by the eyecare provider.13 While the signs, symptoms and visual outcomes may be different between each entity, their overall systemic and neurologic significance and management are the same and should be reviewed.
 |
|
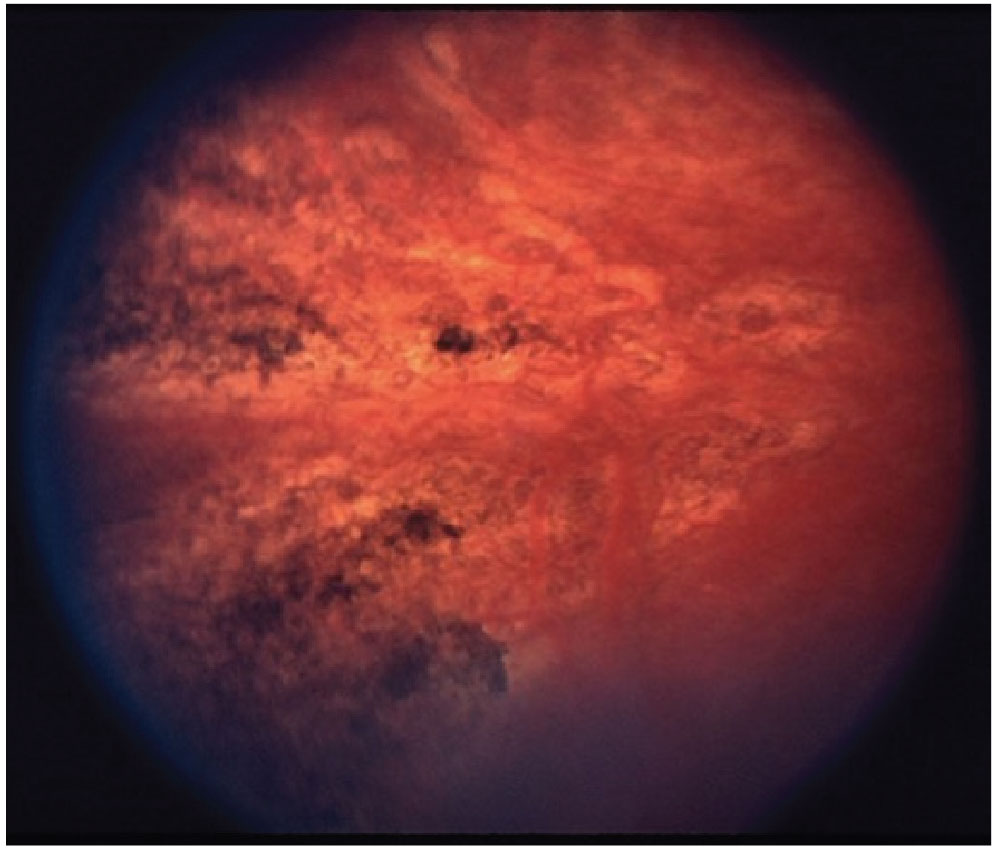
Fig. 2. Siegrist streaks, a rare manifestion of hypertensive choroidopathy. Photo: Retina Image Bank. Click image to enlarge. |
Understanding the protocols for timely referral of these patients may be lifesaving, as risks for stroke are at the greatest within the first few days of onset of these signs or symptoms.13
• TMVL is a temporary episode of vision loss in one eye that can last a few minutes to hours followed by spontaneous recovery. TMVL of an ischemic or vascular nature is caused by impaired blood flow to the retina due to either a vaso-occlusive or embolic event from atherosclerotic plaques; this is sometimes referred to as a retinal TIA.11,12 The term amaurosis fugax (from the Greek meaning fleeting darkness) is often used interchangeably with TIA to describe interrupted vision.11
TIAs usually last less than one hour and have a high risk of subsequent cerebral ischemia and stroke—at its highest during the 14 days following initial onset of symptoms.11 It has been reported that 10% to 15% of patients with a TIA will have a stroke within 90 days, with approximately half occurring within 48 hours. Patients who survive the initial high-risk phase have a 10-year stroke risk of approximately 19%. Considered along with other related conditions, these patients present a 43% risk over 10 years of experiencing a stroke, myocardial infarction or vascular death.13
Patients at Risk for Stroke: The Role of the OD1. Identify and establish a relationship with the nearest certified stroke center open 24/7. 2. Offer same-day appointments for patients with acute painless monocular vision loss (transient or permanent). 3. If confirmed diagnosis of vascular TMVL, BRAO, CRAO or Malignant Hypertensive Retinopathy, check blood pressure in office, inquire about additional systemic symptoms, consider GCA. 4. Send patient immediately to nearest stroke center or rapid-TIA ED with a note indicating “Ocular TIA” or “Ocular Stroke.” Inform and educate patient on the urgency of referral. Call the center to warn them that “a stroke patient is on the way.” Workups in the ED to include:
|
Common causes of TMVL include an embolus of the internal carotid artery or from the heart, atherosclerosis, giant cell arteritis (GCA) and carotid artery dissection.12 Cases of TMVL deserve emergent referral for neurologic imaging for stroke prevention and to confirm a diagnosis. Symptoms of vascular TMVL due to a retinal source include episodes of painless blurring of vision (transient visual obscuration) described as a veil, shade or haze that either ascends or descends vertically and darkening or blacking out of vision, lasting seconds to minutes, that spontaneously resolve. Complaints of “zig-zag lines,” “colors,” “heat waves” or “sparkles,” known as scintillating scotomas, are visual symptoms of aura more commonly associated with vasospastic migraines than with ischemia.11
TMVL can occur secondary to arteritic vascular disease, thus it is important to order blood work to test for GCA in patients over the age of 50.11 Those with elevated inflammatory markers, such as elevated c-reactive protein (CRP) or elevated erythrocyte sedimentation rate (ESR) and elevated platelets along with new-onset headaches, jaw claudication and scalp tenderness should be referred immediately for temporal artery biopsy and started on high doses of corticosteroids.11
• Retinal artery occlusions. BRAO and CRAO are caused by acute occlusions of the retinal vasculature that can lead to sudden painless vision loss and result in retinal infarctions.14,15 The main cause of these artery occlusions has been identified and suggested to be emboli from the carotid arteries or the heart, with a range from 3% to 96% from ipsilateral carotid artery disease and 24% to 72% from emboli from cardiac sources.14 Additionally, one study reported that of 375 patients with nonarteritic retinal arterial occlusion, one third had ipsilateral internal carotid stenosis of at least 50% and half had an abnormal echocardiogram.16
 |
| Table 3. Click image to enlarge. |
The ophthalmic artery branches from the distal internal carotid artery at a perpendicular angle and the diameter of the ophthalmic artery is approximately one third of the internal carotid artery. Hence, it is possible for an emboli that causes an artery occlusion to also enter into cerebral circulation, and can therefore lead to cerebral infarctions.14 Consequently, studies have shown that acute cerebral infarctions are frequently observed on diffusion-weighted brain imaging in patients with acute retinal artery occlusions.15
 |
| Table 4. Click image to enlarge. |
Acute ocular treatment for visual recovery may only be possible within a short time window, reported to be within hours of symptom onset.17 The main goal of acute treatment is to attempt to reverse retinal ischemia and restore retinal perfusion.17 This can be done by increasing the blood oxygen content to the retina through vasodilation, such as carbogen inhalation, or reducing intraocular pressure to help increase the retinal artery perfusion through digital ocular massage, anterior chamber paracentesis or IOP lowering medication.17,18 Unfortunately, these acute in-office treatments may not result in significant visual recovery, are widely divergent, do not have strong evidence-based data supporting them and do not treat the underlying, more emergent causes.17,18
 |
|
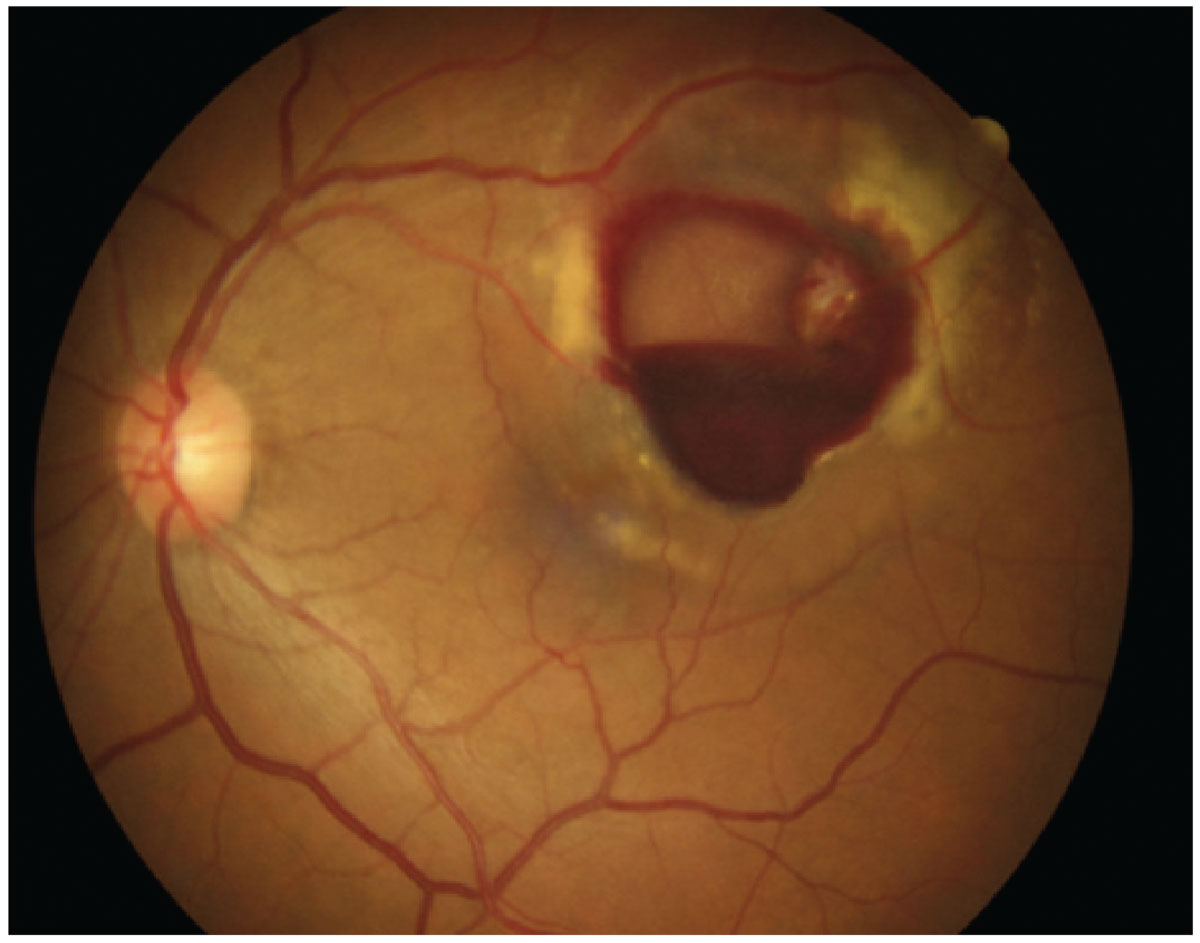
Fig. 3. Retinal arterial microaneurysm. Click image to enlarge. |
Role of the OD
In 2011 and 2013, the addition of retinal cell death, along with brain and spinal cord cell death as attributable factors to ischemia, was included in the definition of a stroke by the American Stroke Association and the AHA.14 Since then, several studies have reported the presence of multiple small cerebral infarctions in up to 31% of patients with vascular TMVL, acute BRAO and CRAOs, further emphasizing that acute retinal arterial ischemia is equivalent to a stroke and must be addressed emergently.14,15,19-23 Findings from the Atherosclerosis Risk in Communities study found that hypertensive retinopathy increases the risk of a stroke two- to threefold, with the Blue Mountains Eyes Study showing a higher risk of combined stroke events in patients with hypertensive retinopathy.24-27
 |
|
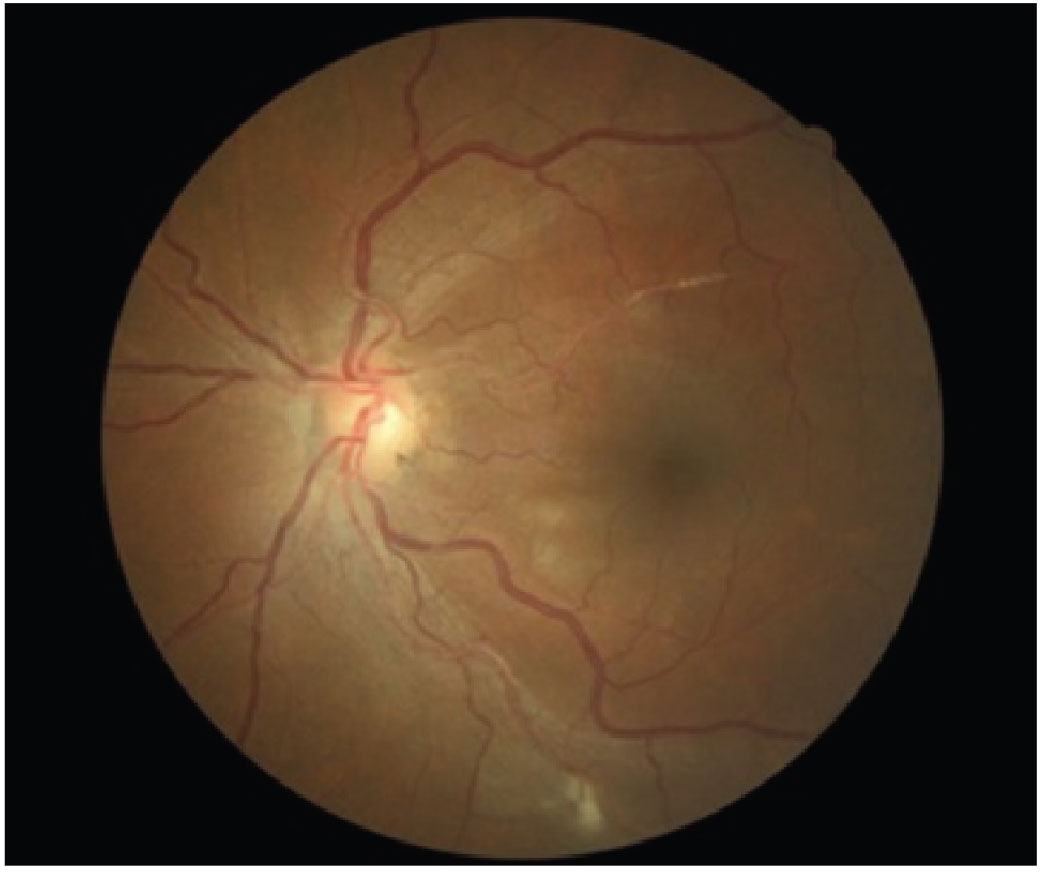
Fig. 4. A patient with grade 3 hypertensive retinopathy. Photo: Diana Mah, OD. Click image to enlarge. |
For patients presenting with severe BP elevation (commonly >200/120mm Hg) with associated bilateral malignant hypertensive retinopathy, an immediate referral to the emergency department is crucial for diagnostic workup and immediate blood pressure reduction to avoid progressive organ failure.28
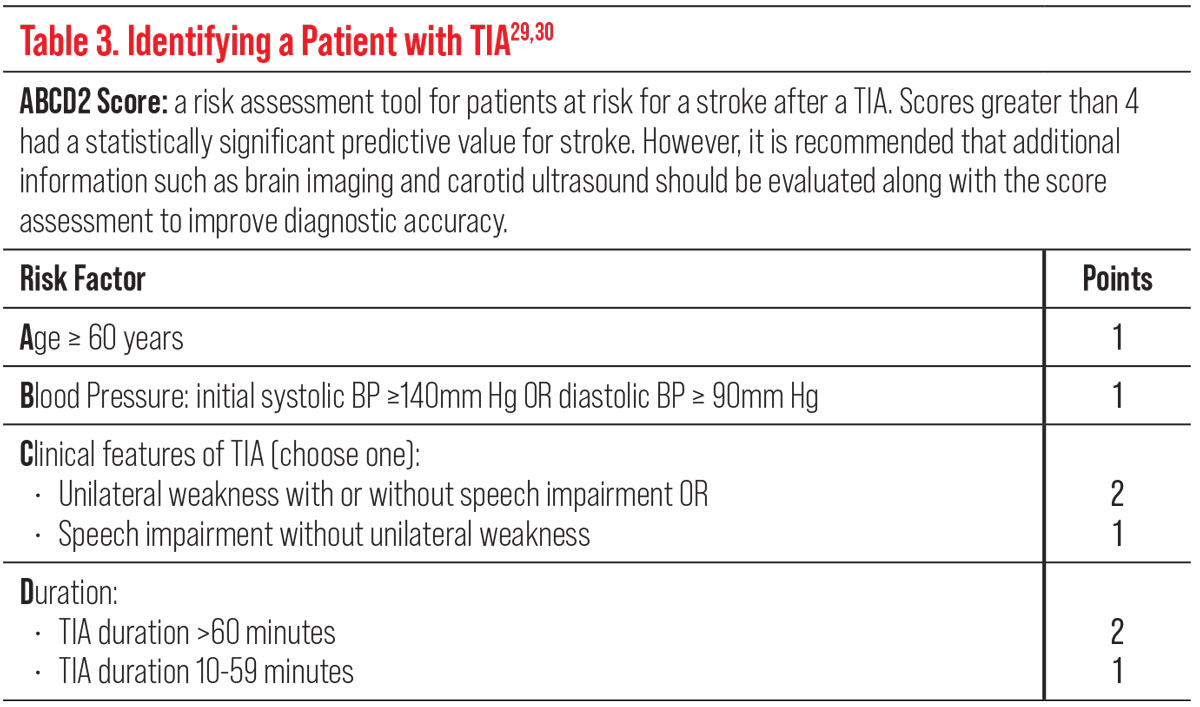
Identifying a patient with TIA, including vascular TMVL, can be difficult but the use of clinical scores such as the ABCD2 can help those at highest risk of subsequent strokes. Higher scores are associated with a higher risk of stroke (Table 3).29,30
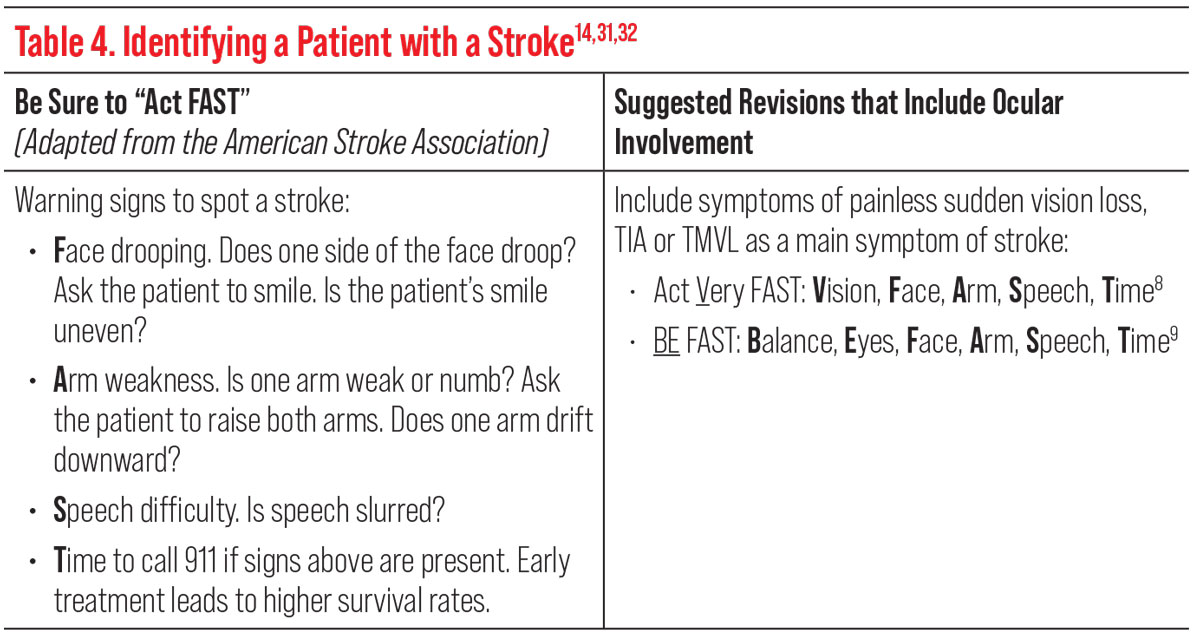
Using the mnemonic “Act FAST,” adapted from the American Stroke Association, can also help identify a patient with a stroke or TIA (Table 4). However, some studies have suggested to add the ocular symptom of sudden vision loss in the acronym to read, “Act VFAST” (act very fast), or to include balance and eyes (“BE-FAST”), either of which could increase screening accuracy and sensitivity (Table 4).14,31,32
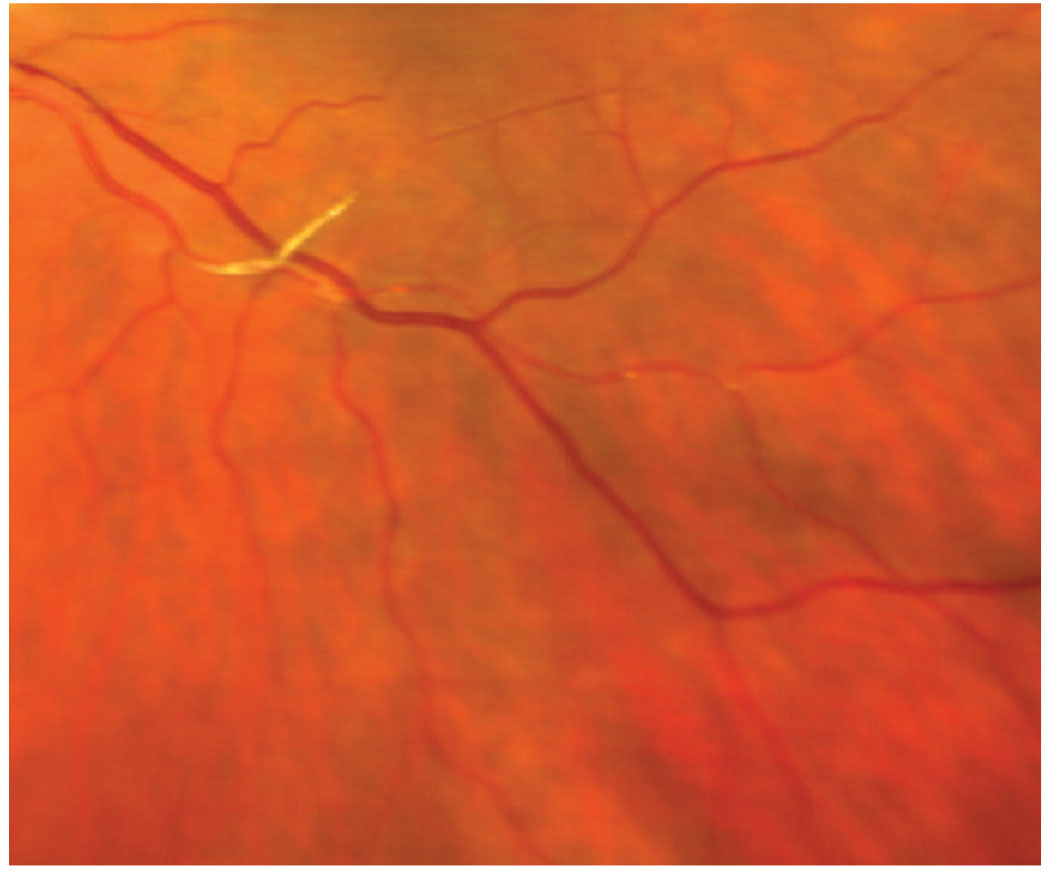 |
|
Fig. 5. Platelet-fibrin emboli. Click image to enlarge. |
Once the diagnosis of TIA or vascular TMVL, BRAO or CRAO has been confirmed, an immediate referral to the closest emergency department with a stroke center or a rapid-access TIA clinic should be conducted. Immediate brain imaging, vascular imaging, cardiac monitoring and lab work should all be completed to rule out GCA. MRI with diffusion-weighted imaging sequences is the preferred brain imaging modality because of its most sensitive and specific modality for detection of early ischemic changes in the brain.15 Multiple small infarctions have been reported to frequently occur ipsilateral to the involved eye on diffusion-weighted MRI of the brain within days of vision loss from acute retinal ischemia.33 Many of these findings were seen in patients with no neurologic symptoms, further reinforcing the importance of immediate brain imaging and stroke work-up in patients with suspected retinal ischemia.15
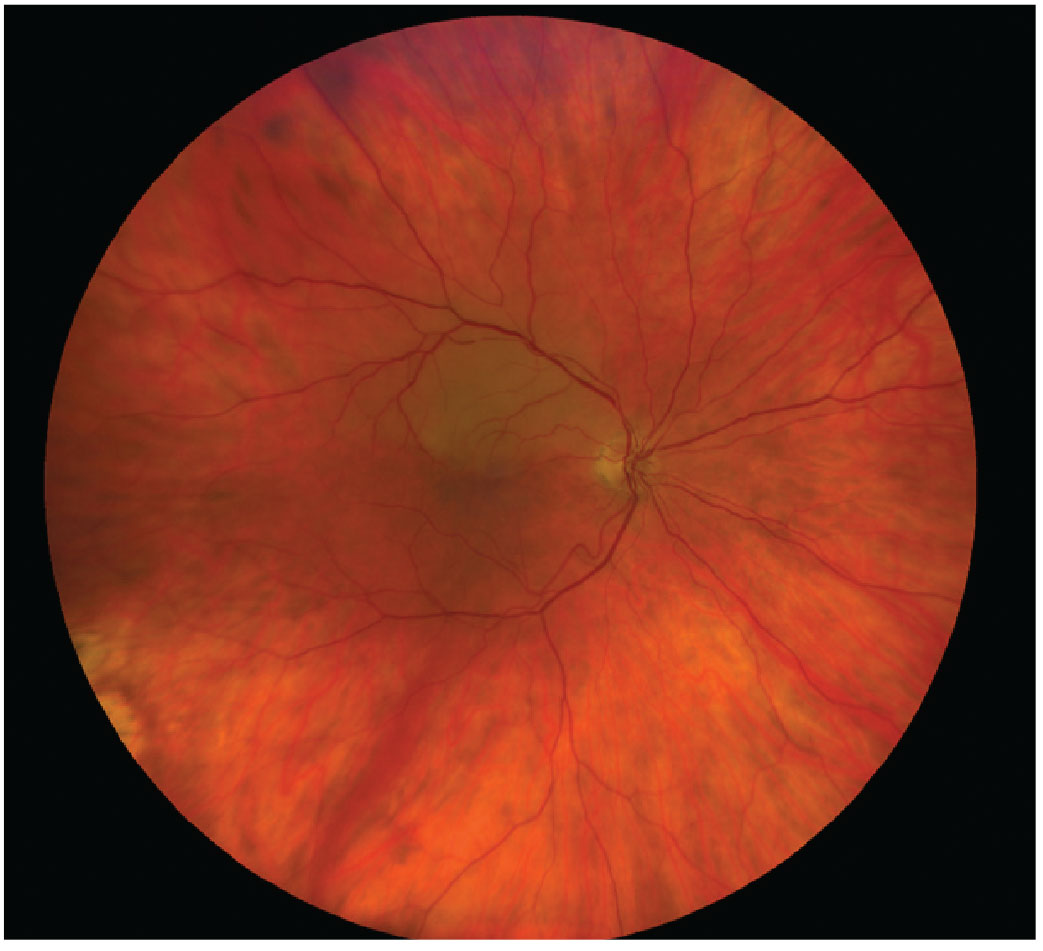 |
|
Fig. 6. BRAO is a form of acute retinal arterial ischemia that is considered both ocular and systemic emergencies requiring proper and immediate diagnosis and management. Click image to enlarge. |
Takeaways
Eyecare providers can play a pivotal role in proper diagnosis and management for patients at highest risk of a stroke by accurately identifying patients who present to an eye exam with an acute TIA, TMVL, BRAO or CRAO. Following guidelines in concurrence with the AHA/American Stroke Association ensures patients will get fast referral to a stroke center and undergo immediate brain imaging.14,15 These quick actions provide the opportunity for early preventative treatments that reduce the risk of subsequent life-threatening stroke or cardiovascular events.14,15
Dr. Komma serves as an externship coordinator and staff optometrist at the Kernersville VA Health Care Center. She graduated from the Pennsylvania College of Optometry and completed her ocular disease residency at the Salisbury VA Medical Center. Dr. Loo is a staff optometrist and eye clinic section chief at the Kernersville VA Health Care Center. She graduated from the Illinois College of Optometry and completed her residency in primary care/ocular disease at the Salisbury VA Medical Center. They have no financial interests to disclose.
1. Centers for Disease Control and Prevention. Hypertension prevalence in the US: million hearts. www.millionhearts.hhs.gov/data-reports/hypertension-prevalence.html. March 22 2021. Accessed September 1, 2022. 2. Centers for Disease Control and Prevention. Stroke. www.cdc.gov/stroke/index.htm. June 14, 2022. Accessed September 1, 2022. 3. Centers for Disease Control and Prevention. Facts about hypertension. www.cdc.gov/bloodpressure/facts.htm. July 17, 2022. Accessed September 1, 2022. 4. American College of Cardiology. New ACC/AHA high blood pressure guidelines lower definition of hypertension. www.acc.org/latest-in-cardiology/articles/2017/11/08/11/47/mon-5pm-bp-guideline-aha-2017. November 13, 2017. Accessed September 1, 2022. 5. Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther. 2018;16(12):879-87. 6. Kiel JW. The Ocular Circulation. Chapter 2, anatomy. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. 7. Hayreh SS. Hypertensive retinopathy. Introduction. Ophthalmologica. 1989;198(4):173-7. 8. Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89(10):1132-45. 9. Pierro L, Pece A, Camesasca F, Brancato R. Hypertensive choroidopathy: a case report. Int Ophthalmol. 1991;15(1):9-14. 10. Ong YT, Wong TY, Klein R, et al. Hypertensive retinopathy and risk of stroke. Hypertension. 2013;62(4):706-11. 11. Jeeva-Patel T, Kabanovski A, Margolin E. Transient monocular visual loss: when is it an emergency? J Emerg Med. 2021;60(2):192-6. 12. Ahmed R, Foroozan R. Transient monocular visual loss. Neurol Clin. 2010;28(3):619-29. 13. Heath Jeffery RC, Chen FK, Lueck CJ. Blackout: Understanding transient vision loss. Aust J Gen Pract. 2021;50(3):136-40. 14. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines. Ophthalmology. 2018;125(10):1597-1607. 15. Lee J, Kim SW, Lee SC, et al. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157(6):1231-8. 16. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116(10):1928-36. 17. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15(1):63-77. 18. Chronopoulos A, Schutz JS. Central retinal artery occlusion-A new, provisional treatment approach. Surv Ophthalmol. 2019;64(4):443-51. 19. Tanaka K, Uehara T, Kimura K, et al. Japan TIA Research Group 2009-2011. Features of patients with transient monocular blindness: a multicenter retrospective study in Japan. J Stroke Cerebrovasc Dis. 2014;23(3):e151-5. 20. Lauda F, Neugebauer H, Reiber L, Jüttler E. Acute silent brain infarction in monocular visual loss of ischemic origin. Cerebrovasc Dis. 2015;40(3-4):151-6. 21. Cho KH, Kim CK, Woo SJ, et al. Cerebral small vessel disease in branch retinal artery occlusion. Invest Ophthalmol Vis Sci. 2016 ;57(13):5818-24. 22. Golsari A, Bittersohl D, Cheng B, et al. Silent brain infarctions and leukoaraiosis in patients with retinal ischemia: a prospective single-center observational study. Stroke. 2017;48(5):1392-6. 23. Tanaka K, Uehara T, Kimura K, et al. PROMISE-TIA study Investigators. Comparison of clinical characteristics among subtypes of visual symptoms in patients with transient ischemic attack: analysis of the PROspective Multicenter registry to Identify Subsequent cardiovascular Events after TIA (PROMISE-TIA) Registry. J Stroke Cerebrovasc Dis. 2018;27(6):1711-6. 24. Henderson AD, Bruce BB, Newman NJ, Biousse V. Hypertension-related eye abnormalities and the risk of stroke. Rev Neurol Dis. 2011;8(1-2):1-9. 25. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. 26. Mitchell P, Wang JJ, Wong TY, et al. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65(7):1005-9. 27. Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134-40. 28. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020; 75(6):1334-57. 29. Quinn TJ, Cameron AC, Dawson J, et al. ABCD2 scores and prediction of noncerebrovascular diagnosis in an outpatient population: a case-control study. Stroke. 2009;40(3):749-53. 30. Fothergill A, Christianson TJ, Brown RD Jr, Rabinstein AA. Validation and refinement of the ABCD2 score: a population-based analysis. Stroke. 2009;40(8):2669-73. 31. Lawlor M, Perry R, Plant GT. Is the ‘Act FAST’ stroke campaign lobeist? The implications of including symptoms of occipital lobe and eye stroke in public education campaigns. J Neurol Neurosurg Psychiatry. 2015;86(7):818-20. 32. El Ammar F, Ardelt A, Del Brutto VJ, et al. BE-FAST: a sensitive screening tool to identify in-hospital acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(7):104821. 33. Biousse V. Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol. 2014;157(6):1119-21. |