Compliance is a leading issue for glaucoma patients on topical medication, which has led many eyecare providers today to seek alternate treatment options, such as minimally invasive glaucoma surgeries, to control patients’ intraocular pressure (IOP). The newest development in this market segment (after Allergan’s Durysta) is another extended-release implant containing a prostaglandin, called iDose TR (Glaukos), designed to continuously deliver 75mcg of travoprost for up to 36 months. The device was approved yesterday by the FDA with a label indication of a single administration per eye to reduce IOP in patients with ocular hypertension or open-angle glaucoma.
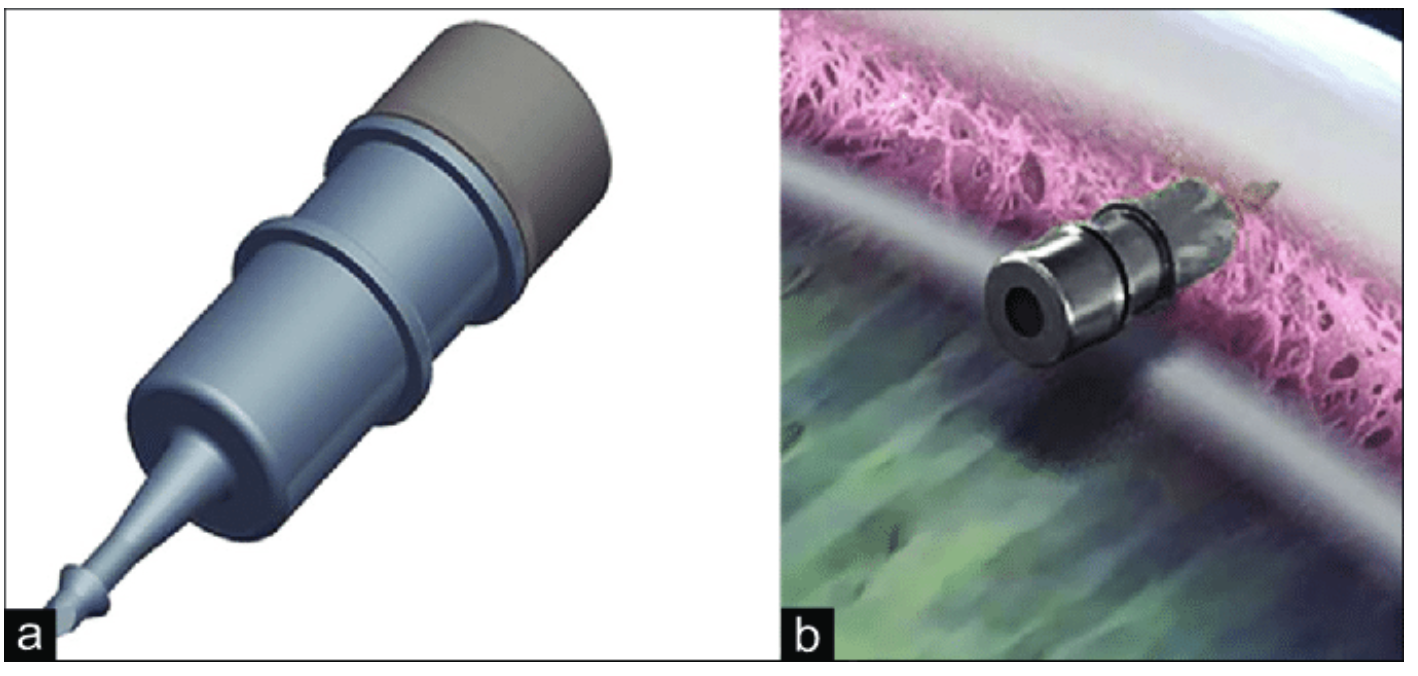 |
| The iDose TR intracameral implant. (a) The iDose TR magnified. (b) Device anchored to the sclera. Photo: Glaukos UK, Ltd. Click image to enlarge. |
Unlike the clinic-based procedure used for Durysta, iDose TR requires implantation in an operating room.
In both its Phase III clinical trials (n=1,150), iDose TR demonstrated non-inferiority over topical timolol in IOP reduction during the first three months; from baseline to month three, IOP reductions in the iDose TR arm were 6.6mm Hg to 8.4mm Hg vs. 6.5mm Hg to 7.7mm Hg in the timolol arm, Glaukos reported in its press release. Of note, iDose TR was not found to be non-inferior to timolol through nine months.
The implant also demonstrated a favorable tolerability and safety profile through 12 months in clinical trials, according to the company. Glaukos reports that at one year, 81% of iDose TR patients had stopped using all IOP-lowering drops, and 98% remained in the trial vs. 95% of timolol control subjects.
Adverse reactions—which included increases in IOP, iritis, dry eye and visual field defects—occurred in 2% to 6% of iDose TR patients, though most were found to be mild, the company reports.
In the first quarter of 2024, Glaukos is planning the commercial launch of iDose TR and has established a wholesale cost of $13,950 per dose (or implant).
For more information, visit www.iDoseTRhcp.com.

