Pain is one of the more common chief complaints when it comes to the eye. Though it is a subjective issue, the anatomy and physiology of pain have been the topics of more research investigations in recent years. We as clinicians treat signs more often than symptoms, so neuropathic pain usually falls outside of our typical order of operations. Time is of the essence in these cases, and when clinical findings do not match patient symptoms, a proper diagnosis can be difficult to make.
This article sheds light on what pain is, the neurobiology involved and what happens when the pain pathway is disrupted and dysfunctional. Keep the following phrase in mind as you work through the content: “Let me explain: pain, to the brain, with no stain.”
 |
|
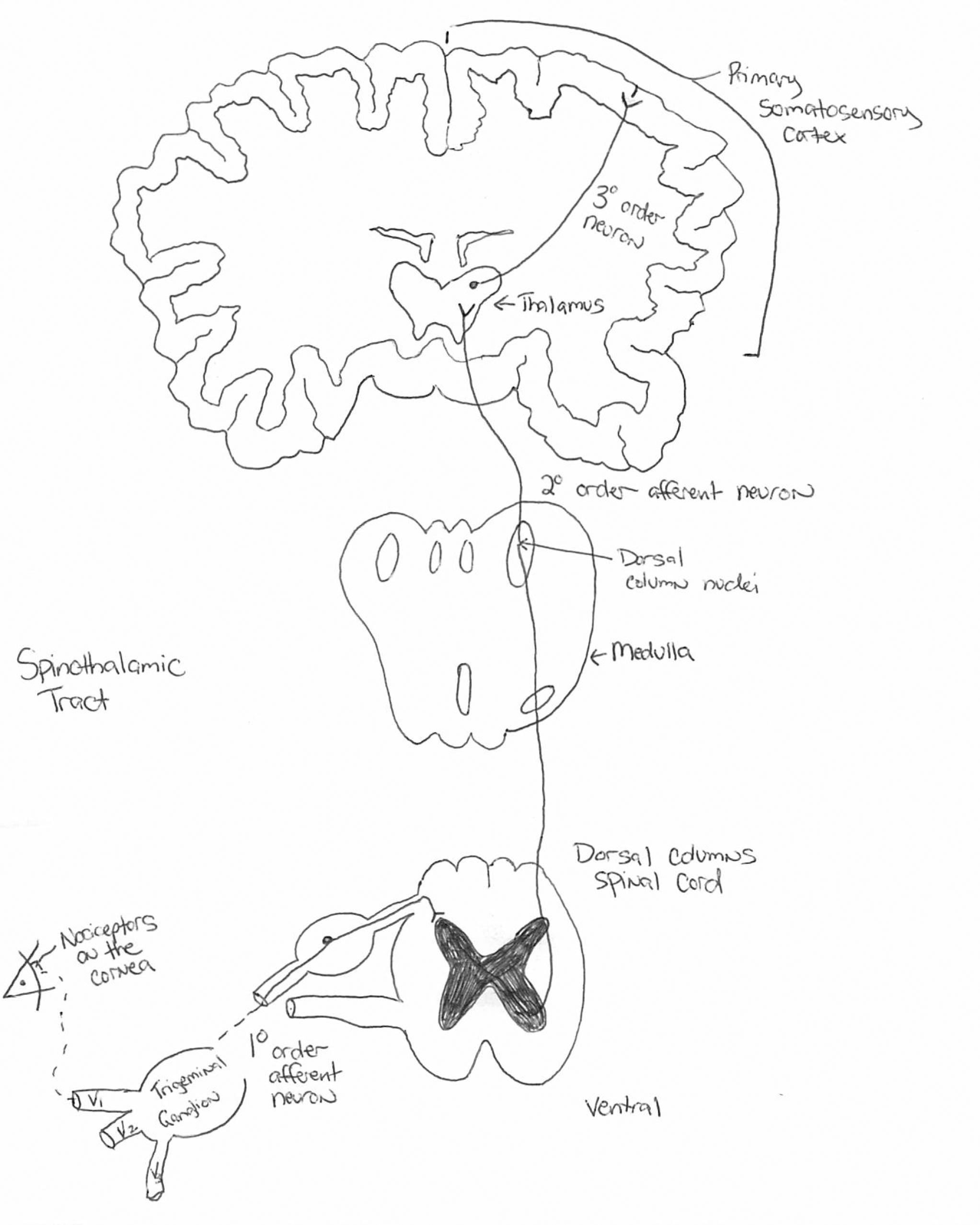
The somatosensory pathway consists of fibers that carry information for pain, temperature, touch, position and vibration. Sensory receptors housed in the dorsal root ganglia project to the dorsal spinal cord, which decussate and extend to the thalamus. Click image to enlarge. |
Pain
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 From there, pain can be broken down into two broad categories: nociceptive and neuropathic.1,2
Nociceptive pain, which is usually transient, arises from the activation of nociceptors—the sensory receptors by which a nerve impulse is triggered—by actual or threatened damaging stimuli. Sites of nociceptive pain generation are located on, but not limited to, the dermis, muscles, cornea, periosteum, endosteum, synovial capsules and parietal pleural peritoneal membranes. These signals detect the conscious perception of mechanical, cutting, stretching, inflammatory, chemical, thermal and traumatic stimuli.
Neuropathic pain, on the other hand, is caused by an insult or disturbance to the nociceptive system, by either a chronic or acute etiology. This leads to overactive pain signaling, where symptoms far outweigh signs on clinical exam.3 Ocular neuropathic pain, therefore, is “a diagnosis of exclusion which refers to the heightened perception of pain in response to normally non-painful stimuli.”2 To put it simply, it is pain with no benefit or purpose.
To the Brain
Pathways for ocular pain originate from a peripheral stimulus that then travels to the central nervous system along the afferent pathway.4 Neural impulses respond to stimuli at the ocular surface—most notably at the cornea, the most innervated area of the body—and then project information from external stimuli to the central nervous system.3,4 This triggers a mechanical and chemical response in the corneal nerve fibers to protect against danger, assist in maintaining a healthy cornea and modulate wound repair.3
The cornea is richly innervated by the ophthalmic division of the trigeminal nerve.3-5 Corneal epithelium-derived innervation from the subbasal nerve plexus has illustrated that the plexus forms a delicate three-dimensional network in the epithelium, originating from the branches of the peripheral stromal nerves.4,5 The tips of the stromal nerves penetrate Bowman’s layer, predominantly in the peripheral cornea, and give rise to long bundles that run from the periphery to the center close to the subbasal epithelia and resemble wavy lines.3-5 One millimeter into the corneal limbus, corneal nerve fibers lose their perineurium and myelin sheaths and are surrounded solely by Schwann cells.3,5 Corneal nerve density increases and nerve diameter thins moving anteriorly through the stroma.3 The overall density of epithelial nerves is greater centrally than peripherally, as the neural network has fully branched out.3,5 Along their course, these long nerve bundles divide into numerous smaller branches that connect, constituting a delicate nerve network within the epithelium.4,5
The generation of a spinal nerve impulse from an external stimulus is called transduction.1,6 This is a signal for “proper pain,” as the stimulation is consistent with the response. The afferent signal travels across the sensory nerve to the relay synapses in the spinal cord, a process called conduction.6 These sensory fibers decussate on the dorsal side of the spine, consistent with the notion that pain on one side of the body is processed on the contralateral side of the brain.6 Once the signal is transmitted in the spine, it then travels up the spinothalamic tract to the thalamus, where the perception of pain originates.1,6
Localization of pain occurs in the sensory cortex of the brain, which tells us where the insult or injury is occurring to elicit a response.6 This relaying of sensory information through the spinal cord, brainstem, thalamus and sensory cortex in the parietal lobe is known as the somatosensory system.1-4,6
Neuropathic pain disrupts the somatosensory pathway, causing “improper pain,” in which a peripheral stimulus has an increased amplitude, duration, frequency or sensitivity to external stimuli.1-4,6 Stimuli are received by receptors and generate an action potential, exciting the afferent sensory cortex of the brain.6,7 Repeated stimulation can cause disproportionate glutamate release from presynaptic afferent nerves, triggering sodium channels in second-order neurons to commence.7 Sensitization to higher-order pain pathways both centrally and peripherally can cause allodynia, or pain due to innocuous stimuli, and hyperalgesia, or enhanced pain perception.7
This discordance can occur at any level of the neural pathway and has many etiologies, including infection, allergy, herpes, trauma, compression, alcohol, vitamin B deficiency and certain neuropathies, all of which can cause a disconnect between signs and symptoms.1-3 Women present with neuropathic findings more often than men, as they are more susceptible to autoimmune conditions that put them at higher risk.2 Diabetes, which has seen an increase in the number of cases over the past decade, is also linked to neuropathic pain.8
The physiology of neuropathic corneal pain helps improve the quality of life for our patients while helping us understand corneal pain at a chemical level. Initiation of the arachidonic acid pain pathway bathes neural cells with cytokines that act on nociceptors, causing the neuron to fire more easily with increased amplitude and duration.1,9 NMDA, another pain receptor, is also increased during bouts of cytokine release, allowing for more impulses to be transmitted.1,9 Sodium channel activation along the neuron triggers neural impulses to fire more easily.9 The most powerful prostaglandin, PGE 2, is released from the inflammatory pain pathway, causing an increase in neural impulses and worsening symptoms.9,10
Chronic inflammation may lead to aberrant connectivity of the nervous system, which may cause irreversible damage.9,10 Pain blockades allow for an increased threshold for stimuli along the pain pathway and reduce neural activation.11
 |
|
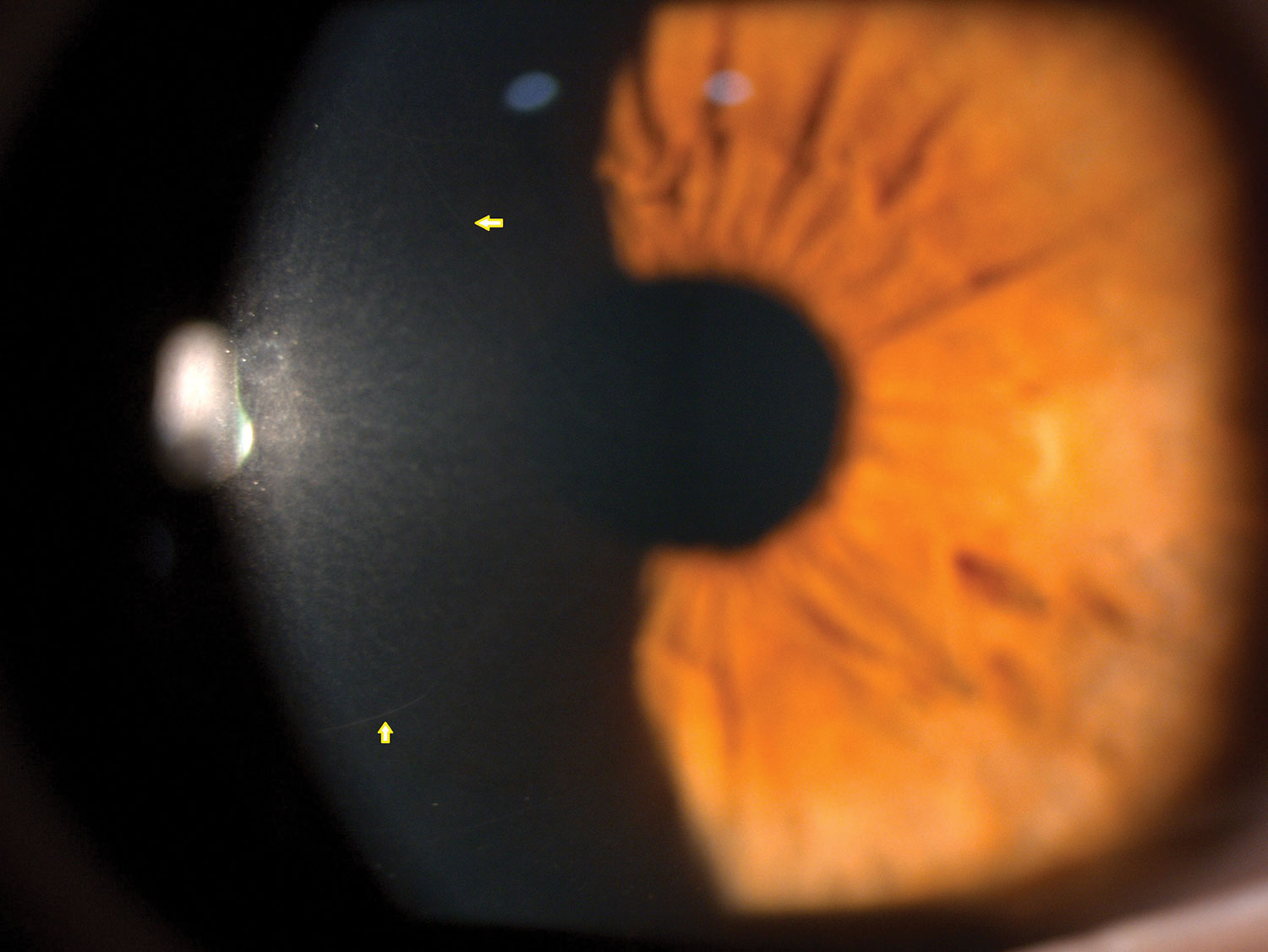
Yellow arrows mark normal corneal nerves in vivo under the slit lamp using specular reflection. Click image to enlarge. |
With No Stain
The debilitating nature of ocular pain compels urgency in management, balancing short-term symptomatic relief with long-term mitigation efforts.
Diagnosis. To properly diagnose true neuropathic pain, we have to confirm neurological impairment. This isn’t as easy as it may sound; the pain is felt, not seen. There is no true clinical diagnostic test for neuropathic eye pain, as these abnormal firings take place at the neurological level and are too small to detect at the slit lamp.12 Sodium fluorescein staining and typical dry eye workups typically come back unremarkable for pathology, with little to no ocular surface disease.1,12
Corneal esthesiometry can be used as a first-line check for abnormalities in the somatosensory pathway.11,13 This test evaluates tactile stimulation of the ophthalmic branch of the trigeminal nerve using a cotton-tip applicator without topical anesthetics.11 Touching the center of the cornea, we can subjectively judge a patient’s reaction and determine nerve function.11,13 Limitations with this method occur because of the all-or-none response to mechanical stimulation that patients are reporting.11 This method fails to quantify corneal nerve sensitivity and may be better suited for neurotrophic corneal disease.
Functional somatosensory testing is also possible with the proparacaine test, which helps localize the initial pain stimulus to either a peripheral or central trigger.1,6,11 Upon installation of anesthetic, if the patient reports full relief from pain we can localize the root cause to a peripheral stimulus. However, if there is no relief or the condition gets worse upon drop installation, we are dealing with a centralized stimulus, a marker for neuropathic pain.1,11
Luckily, there are diagnostic tests and technologies available to visualize corneal nerves, helping us confirm neural disruption. This doesn’t negate the importance of diagnosing comorbidities on the ocular surface, such as dry eye disease, epithelial basement membrane dystrophy and keratitis, to give us a better clinical picture of inflammatory triggers for pain.1-4,6 Managing comorbidities helps with neuropathic pain as we eliminate the underlying causes of cytokine and macrophage release arising from concurrent conditions.1-3,6
Assessment of corneal nerve integrity and morphology is possible with confocal microscopy.1,6,11,13 While there is no objective criterion for the diagnosis of neuropathic pain, with this device we can visualize corneal structures at the cellular level.13 Confocal microscopy acts as a noninvasive biopsy for corneal nerves, allowing for a high-resolution opportunity to pinpoint and confirm nerve damage that may be causing neuropathic eye pain.11,13 Signs of corneal nerve damage apparent with microscopy include nerve beading, nerve loss, tortuosity and increased reflectivity.13,14
Confocal microscopy can also detect microneuromas in corneal nerve anatomy present in neuropathic eye pain but not in dry eye disease.15 Microneuromas are defined as irregularly shaped, terminal enlargements of subbasal nerve endings with variable hyperreflectivity.15 Findings such as these act as an objective, sensitive and specific biomarker for clinical diagnosis.15 Keep in mind, visualization is more difficult when there is concurrent inflammation throughout the cornea from other comorbidities.6,13-15
Management. Therapies for neuropathic pain are designed to help alleviate any dysfunction along the somatosensory pathway.2,7 The best treatment approach for neuropathic eye pain is one that involves multiple modalities to reduce external stimulation to corneal nociceptors and harmful cytokines and chemoattractants that disrupt the normal pain pathway. Neuropathic eye pain has many complex mechanisms that involve the central and peripheral nervous system, so treatment must target all of these impulses to alleviate the signs and symptoms of nerve damage.2,7
The first step in managing neuropathic ocular pain after establishing the proper diagnosis is treatment with preservative-free tears.2,7 Benzalkonium chloride and other preservatives have been linked to the exacerbation of neuropathic pain due to their abrasive impact on the ocular surface. A regimen of non-preserved drops four times per day is recommended as the minimum starting point.2,7,11 Topical corticosteroids such as the ester-based Lotemax (loteprednol, Bausch + Lomb) are particularly useful when initiating therapy.2,7 Gel and ointment forms contain far fewer preservatives (Lotemax ointment is preservative-free) and offer a constant concentration of the drug upon application with fewer side effects.2,7 Lotemax gel can be applied four times per day initially, with a slow taper over six weeks, diminishing the dosage by half every two weeks.7,11
Autologous serum tears are also part of the first-line therapy for neuropathic eye pain due to their powerful anti-inflammatory and restorative properties for neuronal regeneration.2,7,11 Serum tears are formulated from a patient’s own blood after removing red blood cells and clotting proteins.2,7,16 This preservative-free solution serves as another option to relieve signs and symptoms of ocular pain and should be dosed about six times per day to start.2,7
Finally, diclofenac can be used QID to treat ocular surface pain, as it acts as a neuronal potassium channel opener.16,17 The drug suppresses primary afferent neuronal excitatory and firing signals and attenuates excitatory neurotransmitter release and synaptic transmission, creating antiallodynic and antihyperalgesic effects.16,17 Confocal microscopy has shown increased nerve density, reduced beading and reduced tortuosity in vivo in as little as eight weeks.7
The next step in therapy if symptoms have not quelled by this point is supplementation with the use of oral medications. Neurontin (gabapentin, Pfizer) and Lyrica (pregabalin, Pfizer) are the frontrunners, though gabapentin is not considered a controlled substance and therefore is more attractive to prescribe.7 Gabapentin has been proven to relieve neuropathic pain by reducing the amount of inflow of calcium into the neurons, acting to stabilize their response in the central nervous system.2,7 Dosage is 600mg by mouth the first day and then can be increased by 600mg increments to 3600mg (4x900mg daily) maximum.2,7 Common side effects include fatigue, dizziness and confusion, though titration of dosage can be implemented to reduce adverse events.2,7
Doxycycline can also be used to inhibit matrix metalloproteinase, which degrades connective tissue, and increase tear film stability while helping to control ocular surface inflammation and nerve impulses.2,7 Administer this drug at 100mg twice per day for three months and then 100mg once daily for three months before cessation.2,7
Upon implementation of oral medication, it is important to work in conjunction with a pain specialist to help provide relief with potentially stronger medications or more invasive methods.2,7,11 We as optometrists may underestimate the degree of ocular pain these patients experience; however, it remains our duty to improve their quality of life while restoring proper corneal function. Luckily, the aforementioned therapies have been proven to provide structural and symptomatic relief for affected patients.
Takeaways
Corneal neuropathic pain is underrecognized, underdiagnosed and undertreated. Chronic dry eye and neuropathic eye pain may seem congruous but can vastly differ in presentation and treatment.18 More research is needed to fully understand and manage neuropathic eye pain, but at the very least, grasping the complexity of the neuroanatomy and physiology of pain is crucial. ODs are the first line of defense against this debilitating condition and can greatly improve our patients’ quality of life if we take the necessary steps to quell their symptoms.
Maj. Luft practices at Towne Lake Eye Associates in Woodstock, GA. He is a fellow of the American Academy of Optometry. He has no financial interests to disclose.
1. Mehra D, Cohen NK, Galor A. Ocular surface pain: a narrative review. Ophthalmol Ther. 2020;9(3):1-21. 2. Moshirfar M, Benstead EE, Sorrentino PM, et al. Ocular Neuropathic Pain. StatPearls Publishing; 2020. 3. Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31-44. 4. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. 5. He J, Bazan NG, Bazan HEP. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91(4):513-23. 6. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760-72. 7. Goyal S, Hamrah P. Understanding neuropathic corneal pain—gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31(1-2):59-70. 8. Tesfaye S, Boulton AJM, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456-65. 9. Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5-19. 10. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986-1000. 11. Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. 2017;124(11S):S34-47. 12. Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitization in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anesthesia and generalized heightened sensitivity to evoked pain. Br J Ophthalmol. 2017;101(9):1238-43. 13. Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263-85. 14. Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15-47. 15. Moein HR, Akhlaq A, Dieckmann G, et al. Visualization of microneuromas by using in vivo confocal microscopy: an objective biomarker for the diagnosis of neuropathic corneal pain? Ocul Surf. 2020;18(4): 651-6. 16. Pan Q, Angelina A, Marrone M, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017;2(2):CD009327. 17. Galor, A, Levitt RC, Felix ER, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015;29(3):301-12. 18. Jacobs DS. Diagnosis and treatment of ocular pain: the ophthalmologist’s perspective. Curr Ophthalmol Rep. 2017;5(4):271-5. |

