 I recently went to Italy. It was a family vacation and I wasn’t lecturing or interacting with any optometrists or ophthalmologists. However, I couldn’t resist investigating something about eye care while I was there.
I recently went to Italy. It was a family vacation and I wasn’t lecturing or interacting with any optometrists or ophthalmologists. However, I couldn’t resist investigating something about eye care while I was there.
I noticed numerous pharmacies in the areas where I was staying in Rome and Florence, so I decided to drop in to explore. I went to the pharmacist standing behind the counter and simply asked if I could purchase a very common glaucoma medication. I explained that I didn’t have a prescription for this medication. That proved to be no problem. As long as I could provide the correct chemical name, the medication would be sold to me.
After a few moments of inter-language discussion, the pharmacist produced a bottle of a prostaglandin analog for sale to me—and the price, even in Euros, was actually quite reasonable. (I then had the awkward task of explaining that I didn’t actually want to buy the medication, but was instead doing research.)
No Rx Needed?

I paid a visit to this Italian pharmacy.
The aspect of this exchange that I found most interesting is that a doctor’s prescription was not necessary to purchase the glaucoma medication. All that a patient would need to do is remember the medication name or perhaps produce an empty bottle to get a refill. Conceivably, a patient with glaucoma who is adherent to his or her medication regimen could go for years between progress evaluations in some countries.
This is very different from the United States approach to glaucoma management; here, patients are reappointed for progress evaluations on a three-to-four month basis—in most cases, dilation, photography, diagnostic imaging and visual field analysis are performed annually, while gonioscopy is done initially and when indicated during follow-up.
We have been taught to closely follow patients with chronic diseases like glaucoma. This way, we can adjust therapy if a patient is worsening. I am not sure how closely patients with glaucoma are followed in Italy, but one would think that patients could be getting worse over potentially long intervals between visits (for those patients getting their medication refills without a prescription). Should we rethink our approach to patient follow-up?
A Comparison of Vision Loss
In a population-based ophthalmologic survey in Ponza, Italy, the prevalence rate of primary open-angle glaucoma (POAG) was 2.51% (compared to 2.1% in the Beaver Dam Eye Study), 0.97% for angle closure glaucoma and 0.29% for secondary glaucoma of any kind.1,2 Researchers believed that the prevalence rates of POAG found in the Ponza Ophthalmological Survey were consistent with the results of other population-based studies in other countries, including the U.S. The Ponza study compared the prevalence of severe vision loss in the selected population over age 40 to the prevalence rate from other studies performed in countries throughout Europe with similar socioeconomic conditions and public healthcare systems. While not specific for vision loss attributed to glaucoma, the prevalence rates of severe vision loss listed in this survey were consistent with those from other European countries. Cataract, glaucoma, degenerative myopia and macular degeneration were the main causes of visual loss.3
The overall prevalence rates for blindness and low vision in Ponza were 0.6% and 2.1%, respectively. Of those with severe visual impairment, 22.2% was due to glaucoma. Glaucoma was the cause of 0.19% of the severe vision loss in the overall population studied. Of the 110 people (out of 411 who completed all 12 years of the study) who were ophthalmologically normal at baseline, one developed bilateral visual impairment and another developed monocular visual impairment from glaucoma throughout the study.4
It doesn’t seem that the prevalence of glaucoma is different in Italy compared to the U.S. or other European countries, nor is glaucoma a greater cause of severe vision loss in Italy compared to elsewhere.
In terms of vision loss from glaucoma, the Beaver Dam Eye Study showed that glaucoma wasn’t associated with a clinically significant loss of visual acuity.5 Proyecto VER, the large scale research study of visual impairment among Mexican-Americans, found that glaucoma accounted for 0.15% of visual impairment overall, compared to 0.19% in Ponza.6
So, it appears that the prevalence of glaucoma and visual impairment from the disease is similar between Italy and the U.S.
A Timeline for Monitoring Progression
The main reasons for following patients at relatively short intervals are to identify those who are not adhering or responding to therapy, to locate related risk factors (which might indicate a patient is at risk of worsening) and to find those patients who are actually progressing.
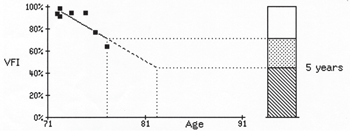
Visual field trend analysis on a treated glaucoma patient. The visual fields for the first several years were stable, but the latest fields show a rapid deterioration in a long-term progressor that would have been missed if not for close follow-up.
There is a strong correlation between poor adherence or persistence with glaucoma medical therapy and poor attendance at office visits or poor understanding of the visual consequences of not using medications.7 Patients who frequently skip their appointments or otherwise have little contact with their doctor and those who don’t understand the negative consequences of vision loss from not using prescribed therapies are most likely to have poor adherence and persistence.
Periodic progress evaluations serve to monitor IOP control, solidify the doctor-patient relationship and afford more opportunities for patient education about the disease. Additionally, pre-set appointments and telephone reminders can help improve adherence to therapy.8
Many studies have looked at risk factors for glaucomatous progression. The Ocular Hypertension Treatment Study (OHTS), the Collaborative Normal Tension Glaucoma Study and the Early Manifest Glaucoma Trial all saw that the development of an optic disc hemorrhage was strongly predictive of progression.9-13 Also, the development or presence of exfoliation syndrome was noted to be a risk factor for progression.11
Clearly, progress evaluations at regularly set intervals allow clinicians to monitor for the development of disc hemorrhages and exfoliation syndrome, identifying patients at greatest risk for glaucomatous progression and presenting the option of amplifying therapy for these individuals.
While therapeutic treatment can delay vision loss from glaucoma, some patients progress despite seemingly adequate IOP reduction. Such patients may do well for a long period of time and then show a sudden decline. Also, through cataractogenesis, some patients with POAG may develop pupil block and chronic angle closure, which needs to be addressed through laser iridotomy.
For this reason, patients with glaucoma should be evaluated periodically with visual fields, disc observation, gonioscopy and IOP measurement to ensure that the IOP is at a target level and is appropriate without glaucomatous progression.
Despite the success of patients in other countries who can apparently obtain medications without seeing a doctor for years, I don’t see a need to change our pattern of progress intervals of every three to four months (or up to six months in established, well-controlled patients).
Dr. Kabat will return next month.
1. Cedrone C, Culasso F, Cesareo M, et al. Prevalence of glaucoma in Ponza, Italy: a comparison with other studies. Ophthalmic Epidemiol. 1997 Jun;4(2):59-72.
2. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992 Oct;99(10):1499-504.
3. Cedrone C, Nucci C, Scuderi G, et al. Prevalence of blindness and low vision in an Italian population: a comparison with other European studies. Eye (Lond). 2006 Jun;20(6):661-7.
4. Nucci C, Cedrone C, Culasso F, et al. Incidence of visual loss in the Ponza Eye Study, Italy. Eye (Lond). 2005;19(2):175-82.
5. Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995 Jan;36(1):182-91.
6. Rodriguez J, Sanchez R, Munoz B, et al. Causes of blindness and visual impairment in a population-based sample of U.S. Hispanics. Ophthalmology. 2002;109(4):737-43.
7. Kosoko O, Quigley HA, Vitale S, et al. Risk factors for noncompliance with glaucoma follow-up visits in a residents’ eye clinic. Ophthalmology. 1998 Nov;105(11):2105-11.
8. Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008 Aug;115(8):1320-7.
9. Budenz DL. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006 Dec;113(12):2137-43.
10. Drance S, Anderson DR, Schulzer M, et al. Risk factors for the progression of visual field abnormalities in normal tension glaucoma. Am J Ophthalmol. 2001 Jun;131(6):699-708.
11. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003 Jan;121(1):48-56.
12. Bengtsson B. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008 Nov;115(11): 2044-8.
13. Leske MC. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007 Nov;114(11):1965-72.

