 |
History
A 76-year-old female presented emergently with a chief complaint of “a dark spot” in the visual field of her right eye of one day’s duration. Her ocular history was remarkable for uncomplicated bilateral cataract extraction in 2010. She did not report any recent ocular or head injury. Her systemic history was significant for hypertension, hypercholesterolemia and Type 2 diabetes, for which she was medicated with losartan/HCTZ, atorvastatin and glipizide. She reported no allergies.
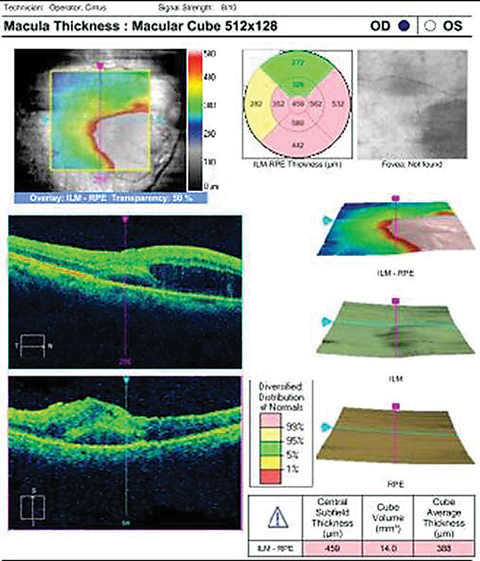 |
| OCT imaging of our 76-year-old patient who complained of a “dark spot” in her vision. Click image to enlarge. |
Diagnostic Data
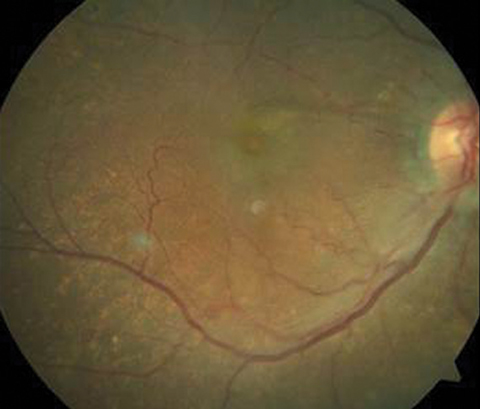
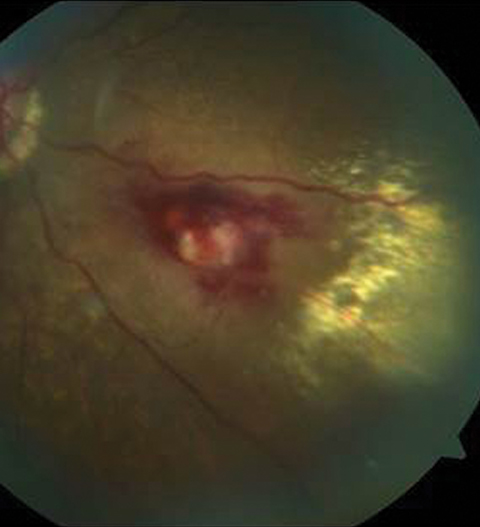
Her entering corrected visual acuities measured 20/120 OD with no improvement on pinhole and 20/30- OS. Her extraocular muscle movements were full and without pain, there was no afferent pupillary defect and the vision could not be improved with refraction, but she was able to position the black spot using the facial Amsler grid. Color vision was unaffected. Biomicroscopy of the anterior segment found normal structures and open angles with Goldmann intraocular pressures measuring 13mm Hg OU. The dilated fundus findings are demonstrated in the photographs.
Your Diagnosis
How would you approach this case? Does this patient require any additional tests? How would you manage this patient? What is the patient’s likely prognosis?
 |
 |
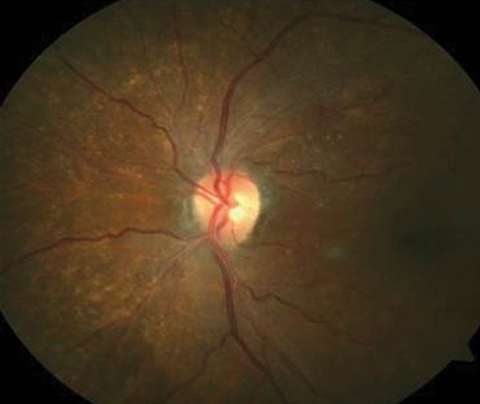 |
| The top two fundus images show the patient’s right eye and the bottom fundus image shows the left eye. Can you identify the cause of the patient’s visual disruption? |
Diagnosis and Discussion
Additional studies that might assist in the documentation and diagnosis of this case include fundus photography to document and follow the lesion, traditional Amsler grid to locate and quantify any resulting scotoma, formal visual field testing and macular OCT to rule out macular involvement.
The diagnosis in this case is exudative fusiform retinal arterial macroaneurysm (RAM) with macular edema. Roberston1,2 first coined the term “macroaneurysm” in 1973, characterizing what he described as acquired focal dilatation of a retinal artery within the first three bifurcations.1,2 The clinical course of each RAM typically follows a course of enlargement, thrombosis, fibrosis and finally, involution.1 Lavin et al3,4 was the first to classify RAM in relation to its main component of exudative, hemorrhagic (both >1DD and central vision affecting) or quiescent.3,4. Quiescent RAMs occur where hemorrhage or exudation exists but the macula is spared and there is no effect on visual acuity.3 Others prefer the anatomical classification which describes RAM by their physiologic nature (fusiform or saccular).3-6
Fusiform RAM demonstrate circumferential widening of the vessel. Saccular RAM show localized “outpocketings” of the vessel wall.3,5,6 Exudative RAM are thought to be fusiform in nature. This is theorized to be secondary to lower perfusion pressure within smaller arterioles further from the disc. Hemorrhagic RAMS are saccular in nature, occurring within the first 3 arteriolar branches.1,3,4 These thinly stretched arteriolar sacs carry a higher perfusion pressure and are more likely to be easily perforated.3 Systoles greater than 200mHg were shown to be more common in hemorrhagic RAMs, which evolve from vessel wall damage produced by greater hydrostatic pressures.1,4,5
Systemic arterial hypertension is the most well-known factor for development of RAMs1-6 with the literature providing a prevalence of 64%-80%.1,3 Greater hydrostatic vessel pressures cause raised wall tension that promotes hyaline degeneration, reduced auto-regulation and hemodynamic stress.3 Additionally, aging arterioles have replacement of smooth muscle fibers in the tunica media and increased intimal collagen, resulting in reduced elasticity and higher susceptibility to vessel dilatation from elevated hydrostatic pressure.3 RAM are more likely to occur at arteriovenous crossings due to their shared adventitial walls. When compromise occurs here, overall structural support of the vessel fails.1,4 The combination of these pathologies, along with age-related factors all work to promote the vascular complication.
The prevalence of RAM is high in patients greater than 60 years old with a gender preponderance for females.1,3,7-9 Typically the presentation is in one eye, with bilaterality reported in less than 10% of cases.1,3,7,8 Researchers suggest both heritable and hormonal causes for the gender prevalence along with a greater older female population.3 Interestingly, RAM and cerebral arterial aneurysms share similar characteristics such as size, structure, pathogenic factors, as well as a similar age of onset.1,3
Differential diagnoses include Coat’s disease, Leber’s miliary aneurysms, angiometosis retinae and capillary microaneurysms (MAs). The first three may share the same exudative clinical appearance but vary greatly epidemiologically. Whereas Coat’s disease, Lebers miliary aneurysms and angiomatosis retinae occur mostly in young & healthy males. The vessel dilatations seen in Coat’s disease where there are multiple venous and capillary saccular outpouchings help to differentiate this from RAM.1
Angiometosis retinae often presents more peripherally and demonstrates greater vascular tortuosity.9 Lastly, both saccular and fusiform RAM tend to range from 100-250 mm in diameter whereas capillary microaneurysms measure less than 100mm.1
The clinical and subjective presentation of RAM can vary greatly. Visual disruption depends upon the degree and location of sub-, intra-, and pre-retinal hemorrhaging as well as macular fluid. Since the deposition of serous fluid and exudate tend to be gradual, the visual complaints may not coincide with the initial date of onset. Subretinal hemorrhage and fluid deposition create toxic conditions for the tissue and can result in permanent structural and visual loss, especially if close to central vision.1 Moosavi et al5-7 discovered exudative RAM present with a circinate ring pattern and tend to be temporal in presentation with no difference between superior and inferior quadrants.1,3 Non-vision affecting or otherwise asymptomatic RAM typically are discovered on routine examination with a characteristic Z-shaped arteriole defining the RAM formation.1
Fluorescein angiography (FA) poses life-threatening anaphylactic risks and should be considered only in vision threatening cases, specifically, where visibility of the lesion is greatly obstructed. Determination of partial thrombosis can be seen on FA (incomplete or irregular filling of the aneurysm).1,8 It is also common to see arteriolar narrowing both proximal and distal to the area of the RAM.1,7 Additionally, it is not uncommon to observe microvascular abnormalities surrounding the RAM with a wider capillary free zone, capillary dilatation and non-perfusion along with microaneurysms and intra-arterial collateral vessels.1,10 Indocyanine green (ICG) angiography possesses an absorption and emission spectra near infrared allowing the dye to be visualized through dense hemorrhages, showing greater visibility of otherwise obscured structures.1,11 Optical coherence tomography has been used to confirm the presence of macular edema, serous retinal detachment and to assist in monitoring the response to photocoagulative treatment.1,12
OCT angiography (OCT-A) is recent, non-invasive advent in retinal imaging. OCT-A uses split spectrum amplitude decorrelation (SSAD) software to provide visualization of blood flow in four separate layers of the retina. The different layers include the superficial vascular plexus, outer retina, deep retina and choriocapillaris—something FA and ICG cannot offer. Given its utility, OCT-A has been used for 3D topographical localization of multiple retinal pathologies.13 In cases of RAM, Doppler OCT can be used in conjunction with OCT-A to allow for exact localization and to discern thrombosed blood and blood flow volume.13, 14 This provides a utility in long term follow-up care to evaluate the need for laser photocoagulative treatment options if deemed necessary. The limitations to OCT-A include a small restricted area for imaging, long duration of fixation needed by the patient to reduce motion artifacts (6.6s)14, and does not allow for depiction of breakdown in the blood-retinal-barrier as seen in FA leakage.13,14 Additionally, the OCT-A only images blood flow making capture difficult when imaging partially thrombosed areas. OCT-A does not serve as a replacement to FA or ICG but can be a safer alternative with no adverse reactions and better visualization of morphology and activity in indicated cases.13
In asymptomatic cases, RAM can be monitored for 1 month and then at 3 months intervals thereafter until spontaneous involution occurs. In cases of direct macular involvement some form of intervention is indicated. There is currently no established protocol and many types of interventional techniques are available.9 In cases of pre-retinal, intra-retinal and vitreous hemorrhage, spontaneous resolution of the hemorrhage can occur but should still be closely monitored within the first 3 months.3,9
The macular treatment options available include argon laser photocoagulation to adjacent tissue, sub threshold laser treatment to the lesion itself, anti-vascular endothelial growth factor (VEGF) injection therapy, pars plana vitrectomy for visually significant vitreous hemorrhage and pneumatic contained hemorrhage displacement for non-clearing hemorrhages captured by the posterior hyaloid.9
Argon laser photocoagulation can be focused on the RAM itself to speed up involution (direct treatment) or focused on adjacent tissues (indirect application) to decrease surrounding leakage. Complications include lesional disruption, arteriolar occlusion, retinal traction, capillary dropout and sub retinal scarring. Given there are no large prospective trials to date, the data used for decision making has been garnered from smaller case series providing mixed results as to the overall benefits. Recently, the practice of sub threshold laser treatment (STLT) has shown the ability to decrease the complications of traditional laser therapy by its reduced exposure.9 Photodisruptive Nd:YAG laser application to pre-retinal hemorrhages have been shown to be beneficial in membranotomy procedures. The focal disruption of the ILM allows for increased hemorrhage absorption time by the vitreous.9 This method allows for an increased visual recovery time, increased visualization of the retina and reduces the complications of stagnant blood in the tissues (in this case, focal photocoagulative laser was employed to reduce leakage given its macular involvement).3,9
In cases involving the macular area with retinal edema, anti-VEGF injection therapy can be used for faster resolution. Cho et al15 treated involved eyes with symptomatic macular edema and or RAM hemorrhage with bevacizumab (Avastin®, manufacturer, city, state). Data suggested an increased resolution time but with no statistically significant endpoint in acuity recovery.15 Pichi et al16 in their case series using treatment with intravitreal bevacizumab found 94% closure after the second injection and 100% complete resolution 4 weeks after the third injection.16The mechanism by which anti-VEGF injection assists in the resolution of RAM is not entirely understood. It is hypothesized that VEGF acts to increase endothelial amounts of nitric oxide causing vasodilation. Anti-VEGF therapy works to reduce vascular permeability by vasoconstriction, which in turn helps to balance out coagulation and fibrinolysis to promote faster hemorrhage clearance and reduce surrounding edema.9
In cases of non-clearing vitreous hemorrhage, after a 3 month observation period, pars plana vitrectomy (PPV) surgery can be utilized. It’s main purpose is to reduce the time of clearance where macular visualization is necessary for the purpose of understanding the extent of maculopathy.9 The thrombolytic properties of tissue plasminogen activator (tPA) can be used to assist in overall treatment.3 Given its level of invasiveness, many complications can arise following PPV procedures. Those risks include cataract formation, retinal breaks or detachments, active bleeding, macular hole and/or endophthalmitis.9
Lastly, pneumatic displacement with injected perfluorocarbon gas can be performed to displace a hemorrhage within the macular area to reduce visual complications. Patients are instructed to lie prone to assist the displacement for at least a week. During the procedure tPA can also be utilized to assist in hemorrhage clearance along with surgical drainage of sub-macular blood. Similar complications to PPV are seen.9
RAMs are commonly seen in older to middle-aged females with hypertensive disease. They may be discovered serendipitously during a routine exam or produce visually significant symptoms dependent on the amount and location of blood and intraretinal fluid. Cases not producing symptoms can be watched. Cases producing visual disability can be treated with several approaches by retinology.
Dr. Gurwood thanks Andrew Meagher, OD for contributing this case.
| 1. Chow CC, Mieler WF, Mittra RA, Pollack JS. Retinal arterial macroaneurysms. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Mosby, Philadelphia, PA, 2014: 575-9. |

