 |
A 71-year-old Hispanic female presented for a comprehensive eye exam. She had an ocular history of cataracts and dry eye, managed with over-the-counter artificial tears. Her medical history was significant for hyperlipidemia controlled with atorvastatin and osteoarthritis controlled with periodic steroid injections. She reported no history of trauma or allergies.
Clinical Findings
The patient’s best-corrected visual acuities were 20/20 in the right eye and 20/30 in the left with a mild hyperopic prescription. There was no improvement with the pinhole OS.
Her external testing was unremarkable and there was no afferent pupillary defect. Refraction uncovered hyperopia OU with no changes to the acuities. Biomicroscopy demonstrated normal anterior segment tissues, mild nuclear cataracts and normal intraocular pressures measuring 12mm Hg OD and 13mm Hg OS using Goldmann applanation. Her optic nerves were normal and healthy OU.
 |
|
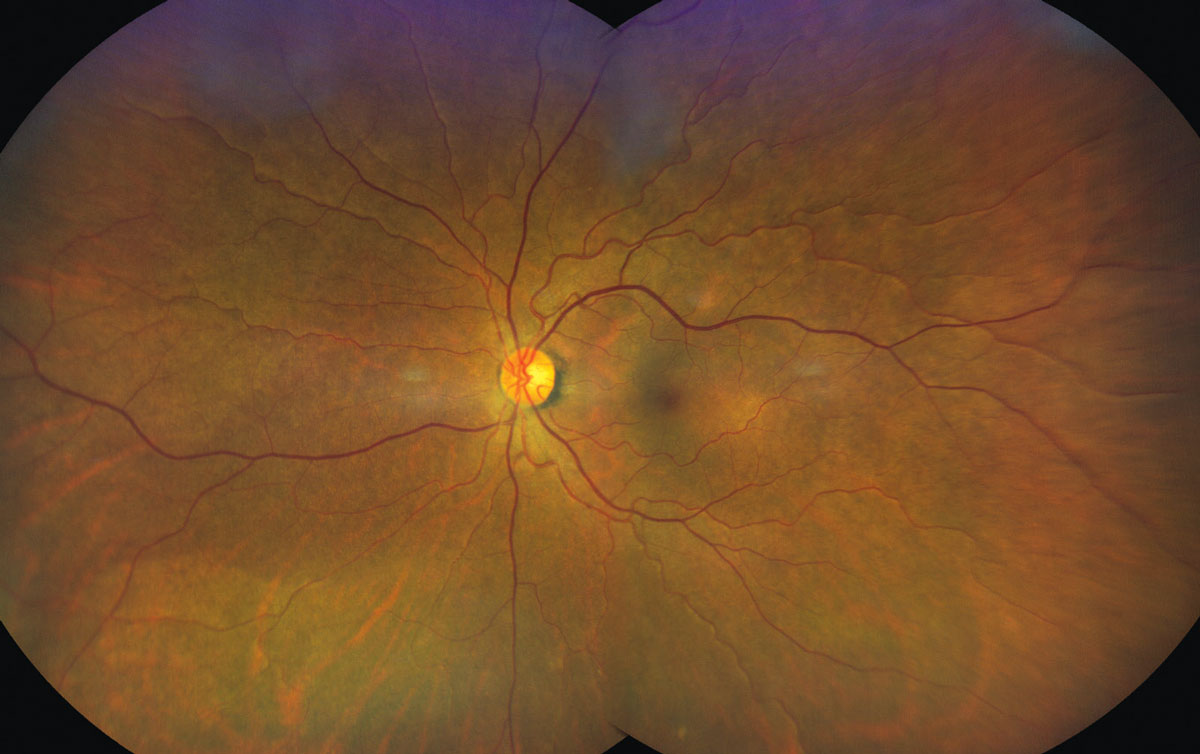
Here’s what the patient’s posterior segment exam looked like. See anything unusual? Click image to enlarge. |
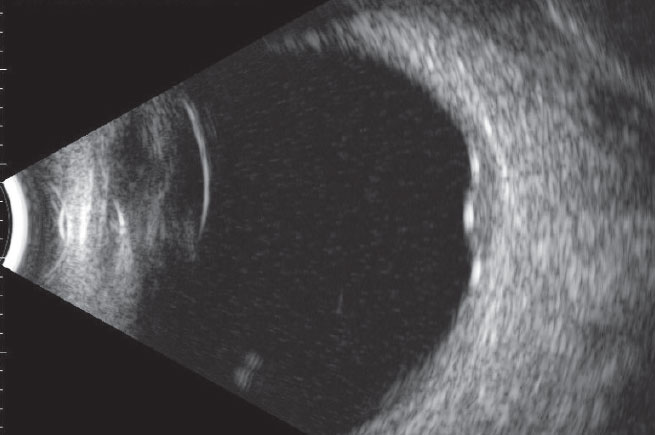
The pertinent posterior segment findings in the left eye are demonstrated in the photograph. B-scan ultrasound of the same eye is also available for review. An OCT scan was also performed, which showed atypical subretinal findings.
 |
|
Does this ultrasonography scan confirm any suspicions raised by the findings shown in the fundus photo? Click image to enlarge. |
Atypical Growth
The diagnosis in this case is circumscribed choroidal hemangioma (CCH), a benign congenital vascular hamartoma (focal malformation that resembles a neoplasm in the tissue of its origin) of the choroid.1-4 It is thought to be present at birth and does not increase with size. It has the potential to leak fluid; this usually occurs in the 4th to 6th decade of life.2,3 It most commonly presents as a unilateral elevated red-orange mass in the posterior pole with no other concurrent ocular or systemic findings. Typically, there are no symptoms.1-5 Its prevalence is difficult to estimate as it is often discovered incidentally upon routine examination or once the patient becomes symptomatic from macular edema.1-3 There can be loss of vision and metamorphopsia when macular edema is present.1-5
The “diffuse” form of choroidal hemangioma is seen in the presence of Sturge-Weber syndrome (a congenital condition affecting the development of certain blood vessels, causing abnormalities in the brain, skin, and eyes) as a diffuse red-colored darkening of the posterior pole in the eye that is ipsilateral to the facial port wine stain (nevus flammeus).2,3,5 Retinal vascular tortuosity may also be observed.5 Although the diffuse form rarely causes macular edema, it does present an increased risk for glaucoma due to associations with elevated episcleral venous pressure and anterior chamber angle malformation.5
The differential diagnosis for a subretinal mass also includes amelanotic choroidal melanoma, amelanotic choroidal nevus, choroidal metastasis (breast, lung, bone, liver), choroidal granuloma, choroidal neovascularization, retinoblastoma, central serous chorioretinopathy, nodular posterior scleritis and Harada’s disease.1-4,5
Multiple forms of imaging are often necessary to definitively diagnose a CCH.2,3 OCT findings are not diagnostic but can show some choroidal thickening and retinal elevation.2,3 Chronic cystoid retinal edema overlying the lesion is often observed. This causes vision loss and metamorphopsia when it occurs in the vicinity of the macula.2,3,5 Fluorescein angiography shows large choroidal blood vessels with early hyperfluorescences that fill the entire lesion and linger up to 20 minutes.2,3,5 Indocyanine green angiography (ICGA) shows similar findings but can reveal the full extent of the lesion, making treatment easier.2,3 B-scan ultrasonography will show a dome-shaped acoustically dense thickening of the posterior choroid with no posterior shadowing.2,3,5 When fluid exudation causes a serous retinal detachment, B-scan is especially helpful as it permits visualizing the lesion.
Consequences and Management
The main causes of vision loss in CCH are macular edema and serous retinal detachment from fluid exudation.2,3,5 Metamorphopsia may also be present when there is macular tilting.1,4 Macular edema is most often found with young patients and those with markedly elevated lesions or findings near the nasal side of the optic nerve.1
Treatment is only needed when there is decreased vision from macular edema or retinal detachment.2 Photodynamic therapy is the treatment of choice, although injection of intravitreal steroid or anti-VEGF agents can also be performed.2,5-7 Low-dose ocular radiation can also be used when needed in cases that are unresponsive to more traditional therapies.2,5,8 Oral propranolol has also been tried, with poor results.2,4
The outcome of treatment is generally good, with 69% of patients achieving visual recovery and 94% achieving resolution of the macular edema.7 Treatment typically has the best results in patients with age younger than 50, pre-treatment VA of less than 20/200 and thinner post-treatment tumor thickness.9
Permanent vision loss may occur when chronic exudation causes atrophic degeneration of the neurosensory retina and retinal pigmented epithelium (RPE).2,5 As a benign lesion, there is no concern for malignancy.6 CCH has no relevant systemic associations that require workup.2,3,5 All subretinal masses deserve suspicion for malignancy.3
We referred this patient to ocular oncology, where she underwent intravenous fluorescein angiography and ICGA, which revealed a rapid-flow vascular mass typical of circumscribed choroidal hemangioma. She was educated regarding this diagnosis and given an Amsler grid for home monitoring. Conservative management was advised, as there was no retinal or subretinal fluid and she was advised to return in six months to ensure stability.
Dr. Gurwood thanks Dr. Nick Karbach for contributing this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
|
1. Krohn J, Rishi P, Frøystein T, Singh AD. Circumscribed choroidal haemangioma: clinical and topographical features. K Br J Ophthalmol. 2019;103(10):1448-1452. 2. Karimi S, Nourinia R, Mashayekhi A. Circumscribed Choroidal Hemangioma. J Ophthalmic Vis Res. 2015;10(3):320–328. 3. Berry M, Lucas LJ. Circumscribed choroidal hemangioma: A case report and literature review. J Optom. 2017;10(2):79–83. 4. Sancho KFCB, Zett C, Gonçalves Júnior I, et al. Effects of oral propranolol for circumscribed choroidal hemangioma. Arq Bras Oftalmol. 2018;81(3):171-176. 5. Augsburger JJ, Anand R, Sanborn GE, Correa ZM. Choroidal Hemangiomas. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Saunders: Elsevier, 2014; 825-9. 6. Campagnoli TR, Medina CA, Singh AD. Choroidal melanoma initially treated as hemangioma: diagnostic and therapeutic considerations. Retin Cases Brief Rep. 2016;10(2):175-82. 7. Boixadera A, García-Arumí J, Martínez-Castillo V, et al. Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmology. 2009;116(1):100-105. 8. Agarwal A, Raghavan V, Rathnadevi R, Rishi P. Treatment of circumscribed choroidal hemangioma using CyberKnife: A viable alternative. Indian J Ophthalmol. 2019;67(5):704–706. 9. Ho YF, Chao A, Chen KJ, et al. Clinical outcomes and predictors of response to photodynamic therapy in symptomatic circumscribed choroidal hemangioma: A retrospective case series. PLoS One. 2018;13(5):e0197088. |

