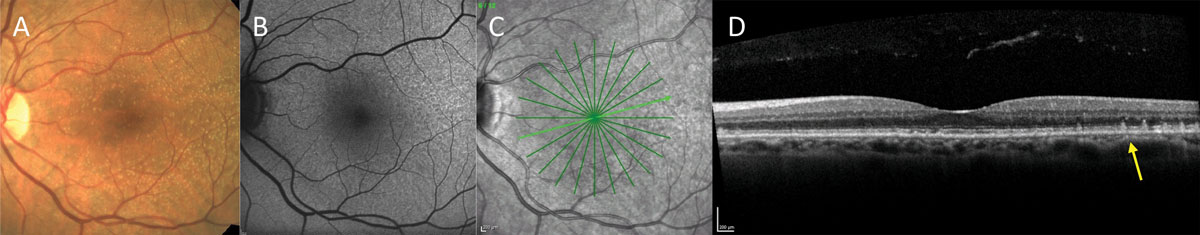
A common finding in AMD, subretinal drusenoid deposits (SDD) are distinct alterations to the retinal pigment epithelium (RPE), characterized by a common uniform phenotype in a wide range of patients. Because of this, researchers from Germany hypothesized a likely hereditary predisposition for development of the structures. As such, they investigated the prevalence and determinants of SDD in first-degree relatives of the patients.
 |
| Experts recommend that AMD patients should encourage their relatives to be screened, given the genetic component of the disease. Photo: Mohammad Rafieetary, OD. Click image to enlarge. |
Individuals with evidence of SDD were recruited, and then invited their relatives. Full ophthalmic exams were done, including SD-OCT, which was graded for presence, disease stage of SDD and percentage of infrared en face area affected by SDD. Genetic sequencing was also done and a polygenic risk score for AMD was calculated.
There was a total of 123 patients and 72 relatives included, with seven of those relatives presenting also with SDD, making the prevalence 9.7%. the study researchers found older age to be associated with presence of SDD and area in the entire cohort, as well as a borderline association of body mass index with SDD presence in relatives. Polygenic risk scores for AMD were typically higher in those with SDD, but this was not statistically significant after multivariate analysis.
The authors noted in their paper on the study that due to SDD’s uniform appearance, they formulated multiple pathogenic hypotheses, ranging from widespread inflammation choroidal vasculature dependencies. However, the mechanisms at play are still undetermined.
They then explained that their observed prevalence rate in relatives was considerably lower than the general population because the mean age of relatives was much lower—usually, the relatives recruited were children of the patients. Two prior studies reported prevalence of 18% in an average of 82 years and 13% in a population aged 77 or older. As such, given the age difference of over 25 years in this study, the authors believe the 9.7% prevalence observed was still relatively high.
Age was the main driver of all detected associations seen in this group. This can likely be explained by the fact that aging can result in various systemic processes that might cause metabolic deterioration and lead to multifactorial disease like AMD. In this study, the association of SDD with age confirms previous study findings outlining the relationship. However, the authors point to only two relatives with SDD who presented with AMD-defining retinal lesions but with small alterations. They suspect that “this may indicate an independency of SDD from AMD (particularly in the early course of disease) and/or suggest that SDD could be a precursor in those at risk. Given the age difference between index patients and relatives, relatives may have been too young to present large drusen and may still develop these in the course of their lives.”
The authors suggest clinical implications of their study include a recommendation to screen relatives of those affected by both SDD and AMD, as well as a need to inform patients and their relatives of preventative measures like smoking cessation, exercise and proper diet. Although more needs to be determined in this relationship, the researchers concluded in their paper that “based on our results, relatives of patients with SDD ought to be closely monitored for retinal alterations, particularly at an older age.”
Mauschitz MM, Hochbein BJ, Klinkhammer H, et al. Prevalence and determinants of subretinal drusen deposits in patients’ first-degree relatives. Graefes Arch Clin Exp Ophthalmol. September 6, 2023. doi.org/10.1007/s00417-023-06221-y. |

