 |
A 42-year-old woman with metastatic cervical cancer presented for baseline ophthalmic evaluation as referred by her oncologist prior to beginning a new systemic therapy, Tivdak (tisotumab vedotin, Seagen and Genmab). She reported mild fluctuating vision and occasional burning. Her best-corrected visual acuity (BCVA) was 20/20 OU with mild inferior corneal staining in each eye with unremarkable posterior segment. A second woman, a 58-year-old with metastatic ovarian cancer, had presented on the same day for ongoing ophthalmic care while receiving Elahere (mirvetuximab soravtansine, ImmunoGen). She had received her first infusion out-of-state and had recently relocated to Florida. She had significant posterior subcapsular cataract in each eye with BCVA of 20/70 OD and 20/25 OS and otherwise unremarkable anterior and posterior segments.
cular treatments, Tivdak and Elahere, represent important advances for disease control in two forms of aggressive, often difficult-to-treat gynecological cancers.1-4 Both therapies have boxed warnings of ocular toxicity and have set protocols for ophthalmic examination and ophthalmic medications during therapy aimed to reduce the risk of ocular adverse events.1-4 Understanding the necessary steps for individuals undergoing or set to begin therapy with Tivdak or Elahere is central for ongoing systemic therapy.
 |
|
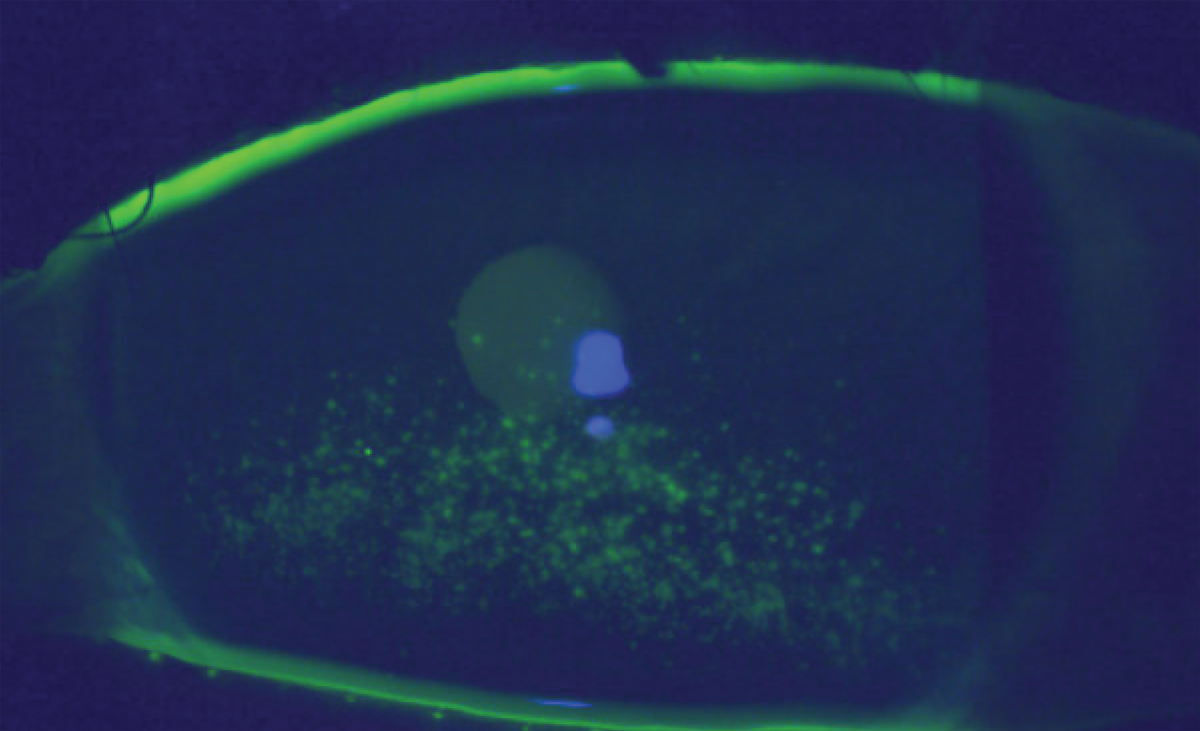
Superficial punctate keratitis in an individual following one infusion of Tivdak. Click image to enlarge. |
Background
Tivdak is indicated as a second-line treatment and beyond for metastatic or recurrent cervical cancer, and Elahere is indicated for the treatment of folate receptor positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer who have received one to three previous treatments.3,4 Both options belong to a new class of cancer therapeutics called antibody-drug conjugates that (in comparison to chemotherapy) have high selectively for cancer cells, which increases efficacy and minimize adverse effects.5 Three segments of the drug—the monoclonal antibody, the cytotoxic payload and the linker—each play a specific role in the drug’s efficacy.5 The monoclonal antibody portion of the drug complex targets specific antigens on the surface of the tumor cell, which leads to uptake of the complex by the cell. The antibody-drug complex is broken down and releases the cytotoxic payload into the tumor cell cytoplasm, ultimately leading to apoptosis.1,5,6
In the Phase II multicenter, single-arm, open-label trial that evaluated the safety and efficacy of Tivdak for treating recurrent or metastatic cervical cancer with disease progression on or after chemotherapy, of the 102 participants, 53% experienced ocular adverse effects.1 The most common ocular adverse events were conjunctivitis (26%), dry eye (23%) and keratitis (11%) with median time to event of 1.4 months.1 The ocular adverse event profile is thought to be due to an off-target event where Tivdak targets tissue factor, the drug’s therapeutic target, present within the corneal epithelium.1
Elahere’s safety and efficacy was evaluated in the Phase II SORAYA study, which enrolled women with folate receptor alpha-high expression with platinum-resistant epithelial ovarian cancer who received one to three prior therapies.2 About 52% of the 106 patients enrolled in the trial experienced blurred vision or keratopathy, with median time to onset of 1.3 months.2 The clinical ocular findings differ from those determined during Tivak treatment. Punctate, often pigmented epithelial deposits and corneal flattening demonstrated on topography have been observed in individuals treated with Elahere.6,7,8 The drug is thought to diffuse into the cornea and disrupt epithelial cell division through the limbal vasculature or via the tear film.8
 |
|
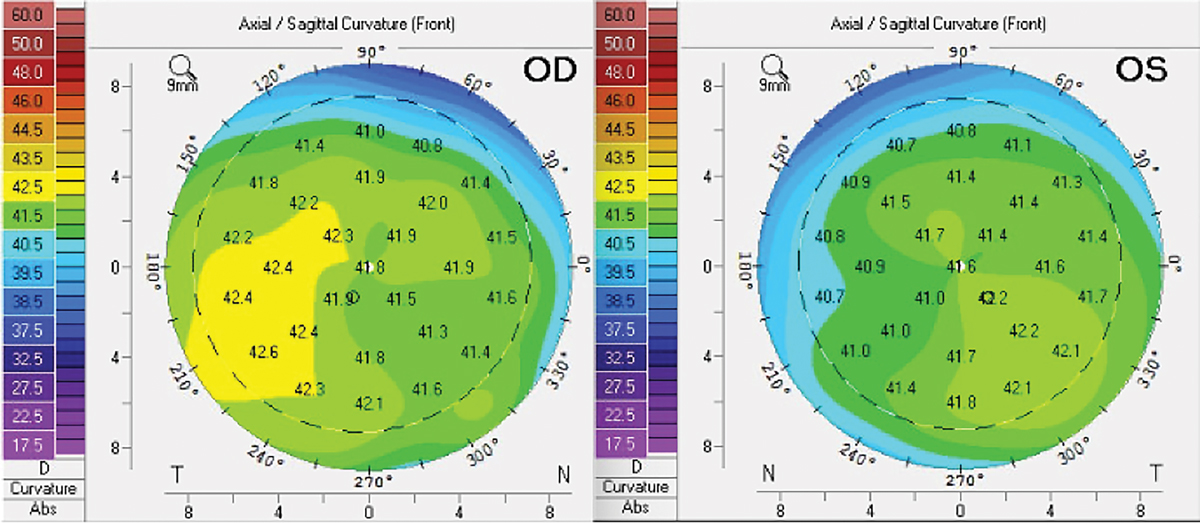
Corneal tomography without evidence of corneal flattening in an individual following four infusions of Elahere. Click image to enlarge. |
Adverse Effects
Eyecare monitoring and treatment protocols were implemented in each trial and are required for either treatment to reduce the likelihood of serious ocular adverse event and ensure management of events if they occur.1-4 Each drug has a specific assessment form that must be completed and made available to the managing oncologist to allow for continued treatment.3,4 If ophthalmic examination is delayed—or the managing oncologist has not received the eye examination report at the time of scheduled infusion—treatment will be delayed. The eyecare plan for each medication considers the risk of ocular adverse effect and includes prophylactic topical ophthalmic treatment to reduce ocular adverse events.
For individuals treated with either Tivak or Elahere, the eyecare plan begins with a baseline assessment of visual acuity and an anterior segment evaluation.3,4 At baseline, we also assess the health of the posterior segment through a dilated pupil, and, for those set to begin Elahere, baseline corneal topography. Considering systemic and topical ocular steroid use during therapy, we also measure intraocular pressure at baseline and at each following examination for patients on either treatment.
For women set to begin Elahere, mitigating ocular adverse events requires a strong topical steroid; for example, prednisolone acetate 1%, starting six times per day the day before each treatment cycle through day four of the cycle, and then four times daily from days five through eight of each cycle and preservative-free artificial tears.2 Contact lenses should not be worn while undergoing therapy.2,3 The topical ocular steroid may help to reduce ocular adverse effects, leading to systemic dose reduction by reducing corneal epithelial cell proliferation and Elahere-corneal microcyst formation rather than through the reduction of inflammation.7 While undergoing therapy, anterior segment examination, VA assessment and completion of the ocular assessment form are required every other cycle (every six weeks) for the first eight cycles.2,3
The ocular adverse mitigation strategy for Tivdak looks a little different. After a patient’s baseline assessment, evaluation and reporting is required prior to each infusion or every three weeks.1,4 The prophylactic therapeutic strategy specifies three drops of brimonidine 0.2% for vasoconstriction instilled into each eye immediately prior to each infusion and topical ophthalmic steroid (we use prednisolone acetate 1%) TID for three days beginning just before the infusion.9 Additionally, preservative-free artificial tears are recommended four times daily. Patients also receive eye cooling pads during the infusion.1,4
Management
For each patient, the drug-specific ocular assessment form was completed with one copy provided directly to the patient to bring to their next infusion appointment and a second copy, along with a summary report, sent to the oncologist’s office. For both treatments, the pre-specified ophthalmic medications were prescribed and reviewed with the patients. We also provided patients with clear printed medication instructions, and advised them to use preservative-free artificial tears four times daily throughout treatment.
The 58-year-old woman has undergone four cycles of Elahere so far without development of ocular adverse event. The 42-year-old woman treated with Tivdak had initial worsening of her keratitis accompanied by a two-line reduction in visual acuity, which was determined to be a grade 2 event. She had reported to be using brimonidine TID OU and prednisolone acetate 1% TID OU daily, rather than only on the day of the infusion (brimonidine 0.2%) and for 72 hours following the infusion (prednisolone acetate 1%). After discussion with her oncology team, the woman on Tivdak continued systemic therapy as planned with an increase of preservative-free artificial tears and reduction of topical ophthalmic medication outlined in the treatment protocol with improvement in corneal staining and return to baseline VA at her most recent visit.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Coleman RL, Lorusso D, Gennigens C, et al. innovaTV 204/GOG-3023/ENGOT-cx6 Collaborators. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicenter, open-label, single-arm, Phase II study. Lancet Oncol. 2021;22(5):609-19. 2. Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA Study. J Clin Oncol. 2023;41(13):2436-45. 3. ImmunoGen. Mirvetuximab soravtansine-gynx (Elahere). Package insert. 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2022/761310s000lbl.pdf. Accessed February 26, 2024. 4. Seagen. Tisotumab Vedotin-Tftv (TIVDAK). Package insert. 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761208Orig1s000lbledt.pdf. Accessed February 26, 2024. 5. Wang Y, Liu L, Jin X, Yu Y. Efficacy and safety of mirvetuximab soravtansine in recurrent ovarian cancer with FRa positive expression: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2024;194:104230. 6. Hendershot A, Slabaugh M, Riaz KM, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep. 2023;47:101155. 7. Matulonis UA, Birrer MJ, O’Malley DM, et al. Evaluation of prophylactic corticosteroid eye drop use in the management of corneal abnormalities induced by the antibody-drug conjugate mirvetuximab soravtansine. Clin. Cancer. Res. 2019;25(6):1727-36. 8. Farooq AV, Degli Esposti S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol. Ther. 2020;9(4):889-911. 9 Supplement to: de Bono JS, Concin N, Hong DS, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumors (InnovaTV 201): a first-in-human, multicenter, Phase I-II trial. Lancet Oncol. 2019;20(3):383-93. |

