By MILTON M. HOM, O.D., FAAO,
and PETER A. SIMMONS, Ph.D., FAAO
Dry, irritated eyes are one of the most common symptoms of patients, and dryness and resulting discomfort are the most prevalent reasons patients give for discontinuation of contact lens wear.1,2 For the practitioner as well as the patient, dry eyes can be a complicated problem, with a number of confusing factors. Many times, we have seen patients present with signs such as moderate bulbar staining and low break-up time (TBUT), but with no complaint of symptoms whatsoever. On other occasions, patients have very marginal staining and normal TBUT, but experience severe dryness symptoms. Because of the inconsistency between signs and symptoms, we feel that regardless of the presence of signs, our primary goal of treatment must be to address our patients" symptoms.
At this point in time, many of our treatments are only partially effective; dry eye cannot be "cured" like we can cure an infection. Primarily what we can offer are palliative treatments such as artificial tears and punctal occlusion. These palliative measures can alleviate symptoms, but many patients obtain only temporary relief, essentially because the cause of their disease is not being addressed.
Pharmacological Products on the Horizon
On the horizon, there are several pharmacological treatments being developed that are designed to address some of the causes of dry eye. One category is the secretagogues such as INS365 (Inspire Pharmaceuticals)3, 15(s)-HETE (Alcon Labs)4, and OPC-12759 (Otsuka Pharmaceuticals)5, which are at various stages of clinical and preclinical testing. These compounds propose to stimulate mucin and aqueous secretion from the ocular surface itself. Other pharmacological approaches under study include use of androgen analogs such as DHEA6 or topical testosterone to stimulate trophic receptors on all of the lacrimal glands, and use of cyclosporine7 or corticosteroid treatment8 to modulate the inflammatory components of dry eye disease. Allergan"s Restasis, a cyclosporine, received FDA approval just last month.
Refresh Endura: An Emulsion-Based Eye Drop
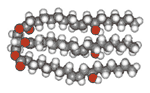 |
| Figure 1. The polar oil found in Refresh Endura. It is principally a triglyceride of ricinoleic acid. The left end is hydro-philic and the right end is hydrophobic |
Emulsions are a mixture of oil and water. A typical example is salad dressing. On the shelf, salad dressing separates into an oil and water layer, and shaking is required to mix the dressing into an emulsion prior to use. The secret behind producing a successful emulsion eye drop with Refresh Endura is the technology in its formulation to produce a stable emulsion not requiring shaking. In the Endura formula, polysorbate-80 has the dual function of demulcent (FDA-approved ocular lubricant) and primary emulsifier, as it has some surfactant properties. Carbomer 1342, a form of polyacrylic acid used as a gelling agent in numerous ophthalmic preparations, is present as a secondary emulsifier. The combination of castor oil, polysorbate-80, and carbomer, combined with a patented process, produce a stabilized emulsion, that appears a uniform pale milky color in the unit-dose vials (see Figures 2 and 3).
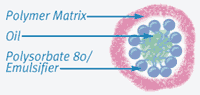 |
| Figure 2. The stabilized emulsion structure shows polar oil surrounded by the emulsifiers polysorbate-80 and carbomer polymer matrix. |
 |
| Figure 3. Refresh Endura is a pale milky color (top), in contrast to standard unit-dose artificial tears. |
Clinical Results with the Emulsion Eye Drop
Prior to its release as a new eye drop, Allergan tested the emulsion product in a 90-day controlled clinical trial in which 73 mild to moderate dry eye patients were treated with Refresh Endura, with measurement of signs and symptoms at 7, 30, 60, and 90 days of treatment. In this group of patients, enhanced stabilization of the tear film was measured as a significant increase in fluorescein tear break-up time: an increase from an average of 5.56 seconds at baseline to 6.96 seconds at day 90 (p=0.006).
The primary symptom variable was a patient-assigned rating of dryness and irritation with possible values of 0 (no symptoms), 1 (trace), 2 (mild), 3 (moderate), or 4 (severe). At study onset, only subjects who rated their symptoms as either 2 or 3 on this scale were enrolled; the average of their scores at baseline was 2.41. The day 90 average rating was significantly reduced from baseline to 1.86 (p < 0.001).
 |
|
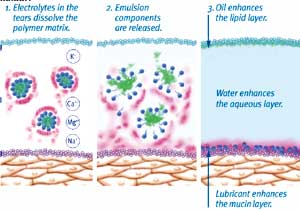
Figure 4. Under the influence of tear film electrolytes, the emulsion is destabilized, releasing the oil into the tear film. |
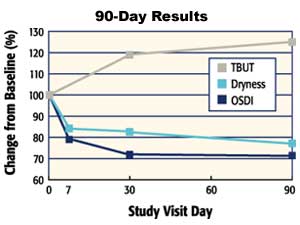
Improvements in the signs and symptoms of dry eye patients of this study are summarized in Figure 6, with changes expressed as a percentage of the baseline values. Of note, some patients reported initial discomfort with application of the emulsion eye drop, with for example 8.2% of patients reporting some level of burning and stinging. These results were obtained with 2 to 4 drops per day. The conclusion of the study is that the emulsion-based formulation of Refresh Endura is safe and effective for treatment of mild to moderate dry eye patients, although it has a different comfort profile than existing artificial tears.
Making the Best Use of the New Drop
 |
|
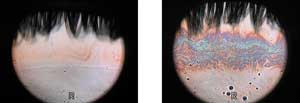
Figure 5. Interferometry images of a normal eye before (left) and after (right) instillation of the emulsion eye drop. The normal thin lipid layer is a uniform pale color, which is greatly enhanced, producing a colored interference pattern across the central cornea. Images courtesy of Dr. Scheffer Tseng. |
For more severe signs or symptoms, we would opt for the enhanced protection of Refresh Liquigel, with 1.0% CMC and higher viscosity as a first-line treatment. If symptoms persist, or if the patient has previously used a variety of artificial tears without relief, then Refresh Endura is indicated.
Dosage may be 2 to 4 times per day as needed, or alternately in some cases concomitant therapy may be appropriate, with the emulsion used BID (morning and evening), with a standard soothing drop such as Refresh Liquigel as a "rescue" during the day. With time, use of rescue medication should decrease, and maintenance on Refresh Endura BID only should be the goal.
Use with Contact Lenses
 |
|
Figure 6. Summary of results of a 90-day controlled clinical study of Refresh Endura. Changes are expressed as a percentage of baseline. Tear stability measured as TBUT improves, and symptoms measured as dryness and OSDI score decrease. |
An Addition to the Arsenal
Emulsion eye drops containing an oil component are an entirely different breed of artificial tears. Because the oil "floats" on the tear film surface following application, its residence time has the potential to be much longer than any standard aqueous tear. Yet because the added oil supplements the existing lipid layer fairly uniformly, there is no visual compromise. This new emulsion eye drop should be an excellent addition to our arsenal in the treatment of dry eye.
References
- Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci 1997; 74(8):624-631.
- Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. Int Contact Lens Clin 1999; 26(6):157-162.
- Foulks GN, Sall K, Greenberg M, Tauber J, Donshik P, Berdy GJ et al. Phase 2 dose ranging efficacy trial of INS365 ophthalmic solution, a P2Y2 agonist, in patients with dry eye. Investigative Ophthalmology and Visual Science 2001;42(4):s713.
- Gamache DA, Wei Z-Y, Weimer LK, Miller ST, Spellman JM, Yanni JM. Corneal protection by the ocular mucin secretagogue 15(S)-HETE in a rabbit dry eye model. Investigative Ophthalmology and Visual Science 2001;42(4):s177.
- Urashima H, Shinohara H, Fujita K. OPC-12759 Ophthalmic suspension increases the ocular mucin of rabbits. Investigative Ophthalmology and Visual Science 2002;43(4): s49.
- Connor CG, Primo EJ. A weak androgenic artificial tear solution decreases the osmolarity of dry eye patients. Investigative Ophthalmology and Visual Science 2001;42(4).
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group [published erratum appears in Ophthalmology 2000 Jul;107(7):1220]. Ophthalmol 2000; 107(4):631-639.
- Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmol 1999;106(4):811-816.
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000;118(5):615-621.

