A mere 10 weeks later, he presents complaining of severe pain, photophobia and reduced acuity. Upon examination, you discover a 1.5mm x 1.75mm lesion in the central cornea, moderate surrounding edema, a significant overlying epithelial defect and stromal involvement. Additionally, there is minimal anterior chamber reaction, 360-degree conjunctival injection and edema/hyperemia of the eyelids. You conclude that the patient has ulcerative keratitis and that you must provide immediate help.
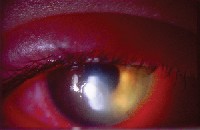 |
| Culture positive Pseudomonas aeruginosa central ulcer with marked inflammation of anterior segment and lids. |
Ulcerative keratitis may present either as an infectious (microbial) or noninfectious (sterile) entity. The infectious form is a sight-threatening condition that requires prompt diagnosis and treatment. Without prompt diagnosis and initiation of appropriate antimicrobial therapy, only half of affected eyes are estimated to heal without some lasting visual disability.1,2
The first step to prescribing sound therapy is to correctly identify the organism responsible for infection.1,3 (If the presenting condition is outside of your comfort zone, immediately refer the patient to a corneal specialist.) Here, well review when treatment is appropriate, how to differentiate what bug youre dealing with and what the most reasonable treatment may be.
Is it Infectious?
To avoid unnecessary treatment, clinicians must differentiate infiltrative keratitis (IK) from true microbial keratitis (MK).
Brien Holden, Ph.D., and his colleagues at the Cornea and Contact Lens Research Unit in Sydney, Australia, coined the term contact lens peripheral ulcer (CLPU) to describe non-infectious contact lens-related infiltrates.4 CLPU is an acute inflammatory condition characterized by a single, small, circular, focal anterior stromal infiltrate in the corneal periphery or midperiphery.4,5
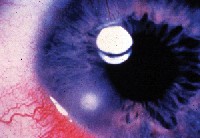 |
| Contact lens peripheral ulcer (CLPU), which respects clinical criteria for non-sight-threatening infiltration. Note location and very focal involvement. |
Although we typically observe CLPUs with extended wear of hydrogel lenses, these infiltrates do not always result from contact lens wear and may occur in patients without a history of contact lens use. Furthermore, there is no ulceration, tissue loss or stromal necrosis and perhaps only subtle epithelial insult without frank epithelial defect (although a full-thickness defect is possible) in these patients.
CLPU is associated with the Staphylococcus species, particularly Staphylococcus aureus.6 Typically, staphylococcal exotoxins trigger an antigen antibody reaction. Leukocytes then accumulate in the subepithelial space located near the immunologically rich limbal vasculature. CLPUs occasionally are painless, and patients often are unaware of their presence. We, too, may not be aware that patients have experienced a CLPU between visits.
Still, whenever lesions are present, we must identify them so that we can initiate treatment if warranted. We also must distinguish ulcerative keratitis from other conditions that can mimic MK. (See How to tell the Ulcers from the Masqueraders.) An infectious etiology should be suspected when the lesion is:
Larger than 2mm in diameter.
Associated with an overlying epithelial defect.
The depth of stromal involvement is greater than 20%, particularly in the presence of stromal loss.
The lesion is located more than 2mm from the limbus.
The borders or margins of the lesion are indistinct or display satellite lesions.
There is marked conjunctival injection and/or chemosis, lid edema and an anterior chamber reaction that is greater than grade 1. The presence of hypopyon suggests the most serious of conditions, as this generally indicates severe inflammation or the onset of endophthalmitis.
The patient experiences pain, photophobia or blurred vision, and mucopurulent discharge is present.
There is a high probability the lesion is infectious if the patient experiences pain, there are one or more stromal infiltrates that measure 2mm or larger and one or both of the following: greater than minimal anterior chamber reaction and mucopurulent discharge.
| How to Tell the Ulcers from the Masqueraders There are several corneal diseases that can masquerade as corneal ulcers. These entities include: Terriens marginal ulceration, Moorens ulcer and furrow degeneration. These are considered inflammatory rather than infectious. Each is characterized by sectoral limbal hyperemia and stromal thinning involving part or all of the limbus. The epithelium remains relatively intact. These conditions are progressive in nature and may be painful or painless. Rheumatoid corneal melt. Increased levels of collagenase in the precorneal tear film of patients afflicted with rheumatoid arthritis can lead to stromal loss/corneal thinning, often in an otherwise quiet cornea. Rheumatoid corneal melts tend to occur in the mid-periphery or periphery. Corneal dellen. This somewhat benign condition generally results from poor corneal wetting or hydration. The condition involves compacted corneal stroma rather than true necrosis. Corneal dellen occurs in quiet eyes and does not involve epithelial loss. Salzmanns nodular degeneration. This uncommon condition may be misconstrued as a corneal ulcer. These gray-white lesions are actually elevations involving the subepithelial space and have no associated inflammatory response. They are chronic in nature and tend to increase in size over many months. They may interfere with overlying epithelial integrity and contact lens tolerance, and may threaten the visual axis and visual acuity. Herpetic eye disease. Known as one of the great masqueraders, herpetic eye disease, or herpetic keratitis, may take on many forms. The rather classic dendritiform ulceration of herpes simplex epithelial keratitis is well recognized. These arborizing lesions demonstrate true excavation of the corneal epithelium and, accordingly, stain positively with sodium fluorescein. The terminal end bulbs, where the replicating virus resides, stain positively with rose bengal. In the case of herpes simplex stromal keratitis, the etiology is believed to be an antigen-antibody inflammatory response within the stroma in contrast to the active viral replication seen in epithelial disease. Accordingly, treatment differs substantially between these two entities. Practitioners differ on the importance of denuding or debulking the viral load via corneal debridement when epithelial disease is present. All, however, agree, that topical antivirals, i.e., Viroptic, are indicated. Topical steroids must be avoided in this population until epithelial recovery is complete. Conversely, in stromal disease, topical steroids are indicated to control this immune-mediated presentation.R.A.R. |
Finding the Pathogen
The virulence of the offending agent dictates what therapy is necessary to eradicate the organism and facilitate recovery. One study reviewed 524 positive cultures and found that 267 corneal ulcers were due to bacteria (51%), 136 were due to fungi (26%) and 121 were due to both bacteria and fungi.7 Of the 430 isolated bacteria, approximately 25% were coagulase negative Staphylococcus, followed by Staphylococcus aureus (23%), Pseudomonas aeruginosa (14%) and Streptococcus pneumoniae (13%). Acremonium species accounted for 40% of all fungi identified, followed by Fusarium species (15%).7
In another study, researchers stained and cultured 427 corneal ulcers in a 13-year year period and found that Staphylococcus aureus was the most common organism followed by Streptococcus viridans, Streptococcus pneumoniae, Pseudomonas aeruginosa, Moraxella and Serratia marcescens.8
To determine the likely pathogen responsible for your patients corneal ulcer, consider the following:
Does the patient wear contact lenses? If the answer is yes, your list of potential microbes must include Pseudomonas aeruginosa, the most common cause of infection in contact lens wearers, though other Gram-negative bacteria have also been identified in these patients.9,10
(Of great importance: Instruct patients to discontinue contact lens wear immediately and to wait at least 24 hours after the infection is completely eradicated before resuming lens wear. Lens polymers and soilage can harbor bacteria and perhaps shield the bacteria from disinfecting agents. Also, the lenses may further aggravate any disruption of epithelial barrier function that has occurred, facilitating invasion by opportunistic bacteria.)
If the patient is not a contact lens wearer, you may be dealing with Staphylococcus aureus, the most common cause of bacterial corneal ulcers in non-contact lens wearers.9
Where does the patient live? Fungal microbes are common in agricultural and tropical climates, such as the southeastern United States, but they are relatively uncommon in the northern and northeastern United States.11 (For more on fungal keratitis, see Therapeutic Review: Is there a Fungus among Us?)
What is the degree of anterior segment inflammation? During your evaluation, carefully examine the entire globe and adnexa when formulating a treatment plan. Concurrent lid disease in a patient with ulcerative keratitis often indicates staphylococcal involvement.
A healthy precorneal tear film can dilute potentially infectious colonies, devitalize less aggressive microbes and inactivate harmful microbes via enzymes and immunoglobulins. Posterior lid disease, however, may significantly alter the quality of the tear film, leaving the ocular surface vulnerable to microbial invasion.
Hyperemic/edematous eyelids may simply reflect the extension of the inflammation when the anterior segment is hot, or may signal a more widespread involvement of the infectious process. Preseptal cellulitis is of particular concern due to the great degree of vascularization of the lids and their communication with cerebral circulation. Check for tenderness to palpation, fever or malaise, and treat aggressively with oral antibiotics. Cephalexin or dicloxacillin for 10 to 14 days are logical choices.
Similarly, anterior chamber reactions may represent uveal involvement and should trigger the use of cycloplegia to treat discomfort, photophobia and discourage synechia.
 |
| Microbial keratitis (MK) with central ulcer and marked stromal loss. Cyanoacrylate glue forestalls perforation; note hypopyon suggesting infectious etiology. |
Traditionally, we have considered culturing to be the standard of care for a suspected bacterial corneal ulcer. A culture tells you what specific pathogen youre dealing with. The reason for positively identifying the pathogen is to confirm that the medications you have chosen are positively effective.
Culturing is still important. However, given the potency and effectiveness of the topical fourth-generation fluoroquinolones, namely Vigamox (moxifloxacin 0.5%, Alcon) and Zymar (gatifloxacin 0.3%, Allergan), culturing is less of a factor. Thats because most organisms are eradicated before the results come back from the lab. (For more on culturing, see Bringing Culture to Your Practice.)
| Bringing Culture to Your Practice To culture corneal ulcers, collect tissue and transfer it to the appropriate media. Some pearls to consider when culturing: Equip your practice with the appropriate instruments. These include calcium algonate swabs, Kimura spatulas, 25-gauge needles or a golf club corneal spud. Keep appropriate media on hand. This includes thioglycolate broth, Saborouds media and blood and chocolate agar plates. Culture the lids and conjunctiva. Many offending agents may reside on the adnexa. When culturing the conjunctiva (both the bulbar and palpebral conjunctiva), culture the deep fornices. Collect any mucopurulent material present by swabbing the area. Try to avoid other culture sites to prevent cross-contamination. Avoid anesthetizing the culture site. Preservatives in the anesthetic drop may affect your yield. The involved tissue is relatively hypoesthetic, due to the degree of inflammation, and may be cultured without significant discomfort. If anesthesia is necessary, single-use ampules of non-preserved proparacaine or tetracaine are available. Do not be overly concerned about removing corneal tissue. Much of this tissue is edematous and infiltrated. As the cornea heals, however, collagen synthesis will replenish the tissue. Culture contact lenses and contact lens cases. The offending agent is often isolated on these as well.R.A.R. |
Studies have shown that monotherapy with topical fluoroquinolones rivals the antimicrobial efficacy of earlier generation antibiotics and may offer patients a shorter duration of intensive therapy.12-15 In one study, however, serious complications, such as corneal perforation, evisceration, or enucleation of the affected eye, occurred more often in patients who used fluoroquinolones than in those who used other topical antibiotics. So, use caution, especially when dealing with large, deep ulcers in elderly patients.12
Prescription of fourth-generation fluoroquinolones to treat bacterial ulcers is not an approved FDA indication. However, this class of drugs is becoming the treatment of choice given their broad spectrum of coverage against Gram-negative and Gram-positive pathogens.
As we are using these agents off-label, there is no one standard regimen, but given the potential adverse effects of keratitis on visual acuity, it is prudent to treat aggressively in these situations. Youll likely need to prescribe one drop of a fourth-generation fluoroquinolone every 15 minutes for the first three hours. For more aggressive infections, one drop every 30 minutes for the first 24 hours may be necessary. In less aggressive situations, one drop every hour for the first 24 hours is adequate.
Infectious agents continue their attack on the host during somnolesence, so we must ensure adequate therapeusis around the clock, if necessary. Some patients may need to instill drops around the clock; others may be adequately covered via loading doses prior to sleep and upon waking or through use of ophthalmic ointments. In some cases, these ointments may be the same as the drop therapy or may be an adjuvant agent, possibly expanding the spectrum of coverage.
Careful follow-up must be employed to determine the appropriateness of therapy in each situation. A general rule of thumb: infections that appear no worse at 24 hours than at initial presentation indicate that youve prescribed appropriate therapy. However, if there is no evidence of clinical improvement by day two or three, you must reconsider the merit of the initial therapy or the diagnosis itself.
We must carefully educate patients about the importance of compliance with our recommended therapy. Although fourth-generation fluoroquinolones have less likelihood of bacterial resistance, we must not let this fact lure us into a false sense of security. We must stress the importance of continuing therapy at an appropriate therapeutic dose to fully eradicate the offending microbe. In many cases, this may translate to one drop every two hours for days two to four, and four times a day for a full week thereafter. Topical antibiotic therapy should not be tapered below the q.i.d. dosage.
Topical antibiotic therapy may be somewhat toxic to the corneal surface. This fact must be weighed against the effect of the pathogen and the treatment deemed necessary to remediate it. Since the infection will produce inflammation and edema, the well-timed addition of a topical steroid, namely when youve achieved therapeutic control and epithelial recovery is complete, may aid in the overall process. The reason: steroids quiet the immune system, suppressing chemotaxis.
Once the topical antibiotic has reached its mean inhibitory concentration (MIC) over a minimal period of using the antibiotic alone, you should determine if inflammation is of help or is a hindrance. Inflammation is intended to separate good tissue from bad. It is only harmful when it prolongs the movement of healing toward overall homeostasis.
In some cases, even though the epithelium may not be completely closed, initiating topical steroid therapy may be appropriate. Often, this may be accomplished with Pred Forte (prednisolone acetate 1%, Allergan) every three to four hours while awake. The recent development of ester steroids, namely Alrex and Lotemax (lotoprednol etabonate 0.2% and 0.5%, respectively, Bausch & Lomb) has offered an improved safety profile, resulting in a decreased likelihood of elevated intraocular pressure or cataract formation.
Eye patching is contraindicated in all of these cases, but you may use a therapeutic soft contact lens if significant pain results from disruption of the ocular surface. Therapeutic bandage lenses provide improved patient comfort, encourage epithelial recovery and potentate drug availability at the site of compromise. Much of the published work about these devices has been based on conventional hydrogel polymers, but more recently silicone hydrogels have gained favor, given enhanced physiological benefits. This is due to increased oxygen permeability, shape retention, mobility, increased oxygen transmission and resistance to desiccation (water loss). The Focus Night & Day lens (CIBA Vision) is specifically approved for use as a bandage lens.
With increasing therapeutic privileges, scope of practice and patient desire for more convenient means for visual correction, so comes a commensurate intensification of professional responsibility. We must arm ourselves with sharp diagnostic skills and be confident in our treatment protocols to provide competent, full-scope care to our patients. What better way to create patient loyalty and practice vitality in these challenging times?
Dr. Ryan is in group practice in Rochester, N.Y., and is clinical associate in ophthalmology at the University of Rochester School of Medicine. He frequently lectures and publishes on anterior segment topics, actively participates as an FDA clinical investigator for many contact lens manufacturers and provides consultative services.
1. Bennett HG, Hay J, Kirkness CM, et al. Antimicrobial management of presumed microbial keratitis: guidelines for treatment of central and peripheral ulcers. Br J Ophthalmol 1998 Feb;82(2):137-45.
2. Jones DB. Decision-making in the management of microbial keratitis. Ophthalmology 1981 Aug;88(8):814-20.
3. Ficker L, Kirkness C, McCartney A, Seal D. Microbial keratitisthe false negative. Eye 1991;5(Pt 5):549-59.
4. Grant T, Chong MS, Vajdic C, et al. Contact lens induced peripheral ulcers during hydrogel contact lens wear. CLAO J 1998 Jul;24(3):145-51.
5. Holden BA, Reddy MK, Sankaridurg PR, et al. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: histopathologic observations on the nature and type of corneal infiltrate. Cornea 1999 Sep;18(5): 538-43.
6. Wu P, Stapleton F, Willcox MD. The causes of and cures for contact lens-induced peripheral ulcer. Eye Contact Lens 2003 Jan;29(1 Suppl):S63-6; discussion S83-4, S192-4.
7. Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea 2004 May;23(4):360-4.
8. Laspina F, Samudio M, Cibils D, et al. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefes Arch Clin Exp Ophthalmol 2004 Mar;242(3):204-9.
9. Neumaier-Ammerer B, Stolba U, Binder S, Feichtinger H. [Corneal infiltrates and ulcers. A retrospective study of 239 eyes] Ophthalmologe 2004 Jan;101(1):33-8.
10. Mah-Sadorra JH, Yavuz SG, Najjar DM, et al. Trends in contact lens-related corneal ulcers. Cornea 2005 Jan;24(1): 51-8.
11. Bharathi MJ, Ramakrishnan R, Vasu S, et al. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol 2003 Dec; 51(4):315-21.
12. Gangopadhyay N, Daniell M, Weih L, Taylor HR. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br J Ophthalmol 2000 Apr;84(4):378-84.
13. Khokhar S, Sindhu N, Mirdha BR. Comparison of topical 0.3% ofloxacin to fortified tobramycin-cefazolin in the therapy of bacterial keratitis. Infection 2000 May-Jun;28(3):149-52.
14. Kosrirukvongs P, Buranapongs W. Topical ciprofloxacin for bacterial corneal ulcer. J Med Assoc Thai 2000 Jul;83(7):776-82.
15. Hyndiuk RA, Eiferman RA, Caldwell DR, et al. Comparison of ciprofloxacin ophthalmic solution 0.3% to fortified tobramycin-cefazolin in treating bacterial corneal ulcers. Ophthalmology 1996 Nov;103(11):1854-62; discussion 1862-3.

