OCT: Eye Care’s All-Purpose ToolThis versatile device has dozens of functions in eye care today. In the January 2023 issue of Review of Optometry, our experts explore its capabilities in full. Check out the other articles featured in this OCT-themed issue:
|
While not as popular or widely used as its posterior segment counterpart, AS-OCT has earned its rightful place as a valuable, adjunctive tool to diagnose and monitor corneal and anterior segment abnormalities. It uses low-coherence interferometry to enable non-contact, in vivo imaging of ocular structures. This technology is available to practitioners as a dedicated stand-alone device or as part of technology that also images the posterior segment.1,2 This feature will highlight AS-OCT’s most common applications in optometric practice.
Indications
AS-OCT technology has evolved since its commercial availability in the early 2000s. Today, these instruments have higher wavelengths to improve penetration depth (especially through sclera and iris), higher resolution, faster imaging acquisition time and a wider field of view. They can image structures as anterior as the tear film and meibomian glands and as posterior as the lens, with certain units even providing 360-degree views of the anterior segment.
Clinically, it has major roles in corneal and anterior segment disease, contact lens fitting and anterior chamber analysis. AS-OCT images are reimbursable by using the Current Procedural Terminology for AS-OCT (92132, scanning computerized ophthalmic diagnostic imaging, anterior segment with interpretation and report, unilateral or bilateral). Payment is bilateral, so no laterality modifier is required for billing. Practitioners should familiarize themselves with the ICD-10-CM diagnosis codes that can be used in conjunction with 92132 (anterior segment).
 |
|
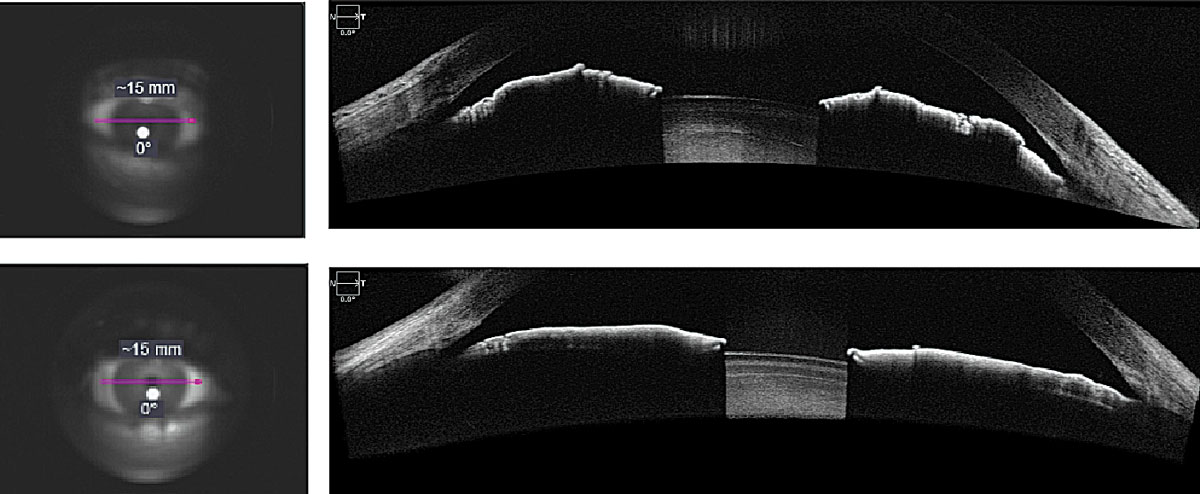
Fig. 1. AS-OCT evaluation of narrow angle (top) and open angle (bottom) using anterior segment angle-to-angle scans. Click image to enlarge. |
Corneal and Anterior Segment Disease
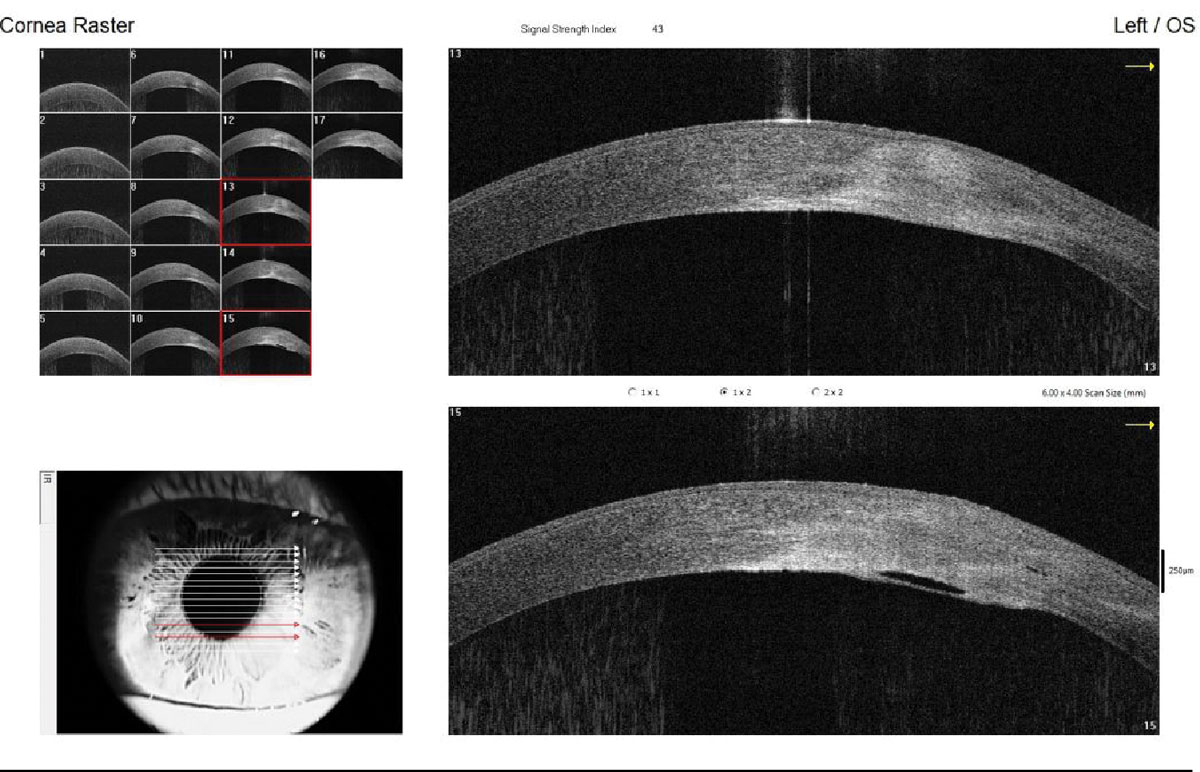
When assessing an AS-OCT corneal image, the first, thin, hyperreflective layer is the tear film. Using the caliper tool, the tear menisci can be measured. Directly underneath the tear film is the hyporeflective corneal epithelium. AS-OCT accurately maps clear and opacified corneas, allowing practitioners to precisely delineate the individual corneal layers: epithelium, Bowman’s, stroma, Descemet’s membrane and endothelium. This can be instrumental when confirming the layer(s) involved for various corneal opacities and dystrophies, such as epithelial basement membrane dystrophy and lattice dystrophy (Figure 3).
Because the cornea is transparent and avascular, borders and landmarks between corneal layers using slit lamp are best appreciated qualitatively with optic section, but AS-OCT has the distinct advantage of quantification (i.e., can measure to the nearest micron). A clinical example where AS-OCT is useful is monitoring epithelial healing from a superficial defect underneath a soft, bandage lens. The defect can be measured and assessed without needing to remove the lens earlier than warranted.3
Its technology can precisely map the depth of corneal and conjunctival abnormalities alike. For example, ocular surface squamous neoplasia, which was historically diagnosed by biopsy alone, has unique diagnostic characteristics visible with AS-OCT (i.e., a thickened, strongly hyperreflective epithelium with abrupt transitions from normal to abnormal epithelium). In contrast, pterygia and pinguecula demonstrate a thin or normal epithelium overlying a fibrous subepithelial lesion.4,5
AS-OCT technology can provide practitioners with important data to help differentiate benign from malignant lesions, including lesion size, internal structures (e.g., cysts), vascularity, layers involved and shadowing and its anterior and poster surfaces. While pathology specimens may still definitively differentiate ocular surface lesions, AS-OCT can still be used to guide appropriate management and confirm adequate treatment depth if excision is necessary.
AS-OCT similarly has applications in the management and diagnosis of infectious, inflammatory and ectatic corneal disease. The thickness of subepithelial infiltrates or corneal scars can be measured with the caliper tool on the AS-OCT and monitored over time as they improve and resolve with treatment.6 In infectious cases, it can distinguish confounding variables such as significant epithelial defects, focal edema and anterior chamber reactions. Microscopic cells in the anterior chamber appear on AS-OCT as discrete, hyperreflective suspended foci. Obtained images can be used to objectively quantify measurements of inflammatory cells and aqueous flare, even when masked behind cloudy corneas.
In ectatic disease, pathologies such as keratoconus were traditionally monitored with corneal topography, pachymetry and eventually Scheimpflug tomography. With AS-OCT, specialists can visualize and track distinguishing features of keratoconus within individual corneal layers. Bowman’s membrane and epithelial thickness maps over the apex of the cone have been highlighted in recent studies as important tools to distinguish non-diseased eyes from manifest, subclinical and forme fruste keratoconus.7 This has important implications for refractive surgery screening, especially in uncertain cases.
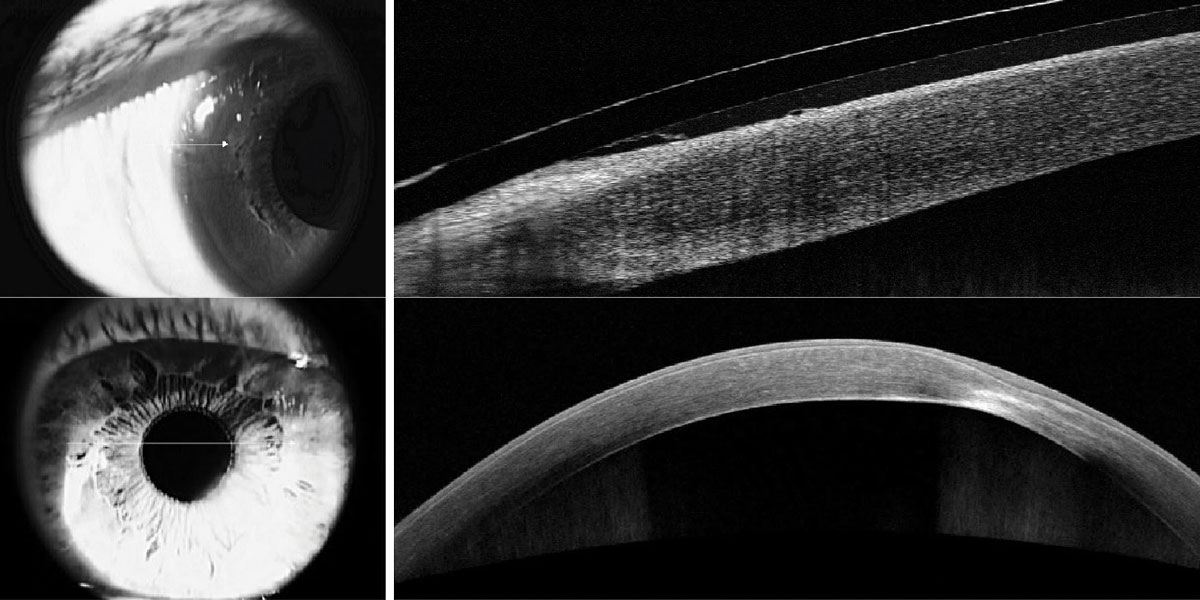
For advanced keratoconus, patients with increased epithelial thickness, anterior hyperreflection at Bowman’s and stromal thinning at the cone are considered to be at-risk for developing corneal hydrops. If corneal hydrops does occur, AS-OCT scans can pinpoint the location of the break in Descemet’s and display the extent of edema present (Figure 2).8 While hydrops is a rare complication, when it occurs the sudden visual loss is distressing and the corneal edema is often diffused, making it difficult to visualize posterior structures. AS-OCT is an invaluable patient education and monitoring tool to follow the resolution of edema over time.
AS-OCT has significant functionality when used preoperatively or intraoperatively for corneal procedures targeted at specific corneal layers, including epithelial debridement, superficial keratectomy and phototherapeutic keratectomy. It can help dictate if a partial corneal transplant, such as deep anterior lamellar keratoplasty, can be performed in lieu of a full-thickness penetrating keratoplasty by confirming that the lesion is located in the stroma alone. It can even precisely locate the LASIK flap interface of previously performed procedures or monitor graft adherence following DSAEK transplantation. By understanding the exact location of the corneal pathology with the help of AS-OCT, clinicians can easily choose and monitor complications of the surgical intervention to maximize outcomes.
 |
|
Fig. 2. Imaging of a resolving corneal hydrops in a patient with keratoconus, highlighting corneal edema and the break in Descemet’s membrane. Click image to enlarge. |
Contact Lens Fitting
One of the most exciting applications for AS-OCT is its role in contact lens fitting for all lens types. The chord and sagittal height measurements provide vital information about peripheral corneoscleral profilometry and sagittal depth.9,10 This allows fitters to measure the sagittal height at specific diameters, allowing for greater precision when empirically designing specialty soft lenses. AS-OCT can assess made-to-order lens fit on eye, guiding modifications to base curve, diameter, prism, optic zone sizes and decentered optics.
A recent study revealed that novel contact lens fitting software built into the AS-OCT may be used in the future to empirically design rigid gas permeable lenses for keratoconus.11 AS-OCT is likewise used routinely to assess hybrid contact lenses without the need for sodium fluorescein by identifying the appropriate amount of vault under the gas permeable component of the lens. It can additionally provide insight into the fit at the junction of the lens and alignment of the soft skirt.
Recently, AS-OCT has been briskly gaining popularity in the diagnostic and empirical fitting of scleral lenses. Even for experienced fitters, the process of fitting scleral lenses can be time-consuming and laborious. Clinicians often need significant pre-fit data (including imaging) and the lens fitting may involve the insertion and removal of numerous scleral lenses, all of which require settling.
AS-OCT has modernized fitters’ abilities to quickly measure clearance over the corneal apex, mid-peripheral corneal clearance, limbal vault and scleral edge alignment accurately and precisely. In fact, technicians and medical assistants can be trained to acquire these images over different trial lenses to further maximize exam efficiency. This is especially crucial for follow-up visits, where patients present wearing scleral lenses with a clear tear reservoir.
While scleral lenses can be evaluated with biomicroscopic viewing alone, a recent study found that novice, intermediate and advanced fitters all tend to overestimate the central corneal clearance using this method.12 With the aid of AS-OCT, fitters can confirm and expedite their clinical assessments to quantify the exact vault in microns. AS-OCT also demonstrates the fitting relationship at the limbus, edges and within the tear reservoir (Figures 3-5). When speaking with lab consultants about these in-office fits, images can be shared directly aiding in the proficiency of modification recommendations. Many optometrists use their devices to assess specialty contact lenses, allowing for more timely, precise and clinically meaningful modifications to complicated fittings.
Anterior Chamber Angle Analysis
AS-OCT can be extremely useful in assessing the anterior chamber. It is an important complement to gonioscopy and can confirm that minimally invasive glaucoma surgeries have targeted the correct anatomy. Practitioners agree that it does not replace gonioscopy, but rather complements it, as AS-OCT is more objective and can be performed under different lighting conditions, which may supplement a practitioner’s understanding of angle closure. It is non-contact, unlike gonioscopy, and requires corneal interaction (which not all patients tolerate well). Gonioscopy also necessitates subjective judgment and substantial training by the observer.
One study found that AS-OCT scans were more reliable in predicting the success of laser peripheral iridotomy than clinical examination by glaucoma specialists.13 While the ZAP trial was focused on determining longitudinal changes in angle configuration in the eyes of primary angle-closure suspects treated by laser peripheral iridotomy and in untreated fellow eyes, there was significant interest in better understanding the anterior chamber and angle anatomy, which the newest AS-OCT technologies may help to do.14
Another major advantage is that AS-OCT allows for quantification of angle parameters, e.g., anterior chamber depth, angle-to-angle distance, angle-to-angle width, pupil diameter, crystalline lens rise and iris thickness. In fact, when quantitative iris parameters were investigated in angle closure disease, increased iris thickness as measured by AS-OCT was associated with a higher risk of angle closure.15 However, while these parameters and quantifications exist, there are not yet strict guidelines of how to manage patients based on these values.
Moreover, AS-OCT has a growing role in imaging iris and uveal lesions in the anterior chamber. While iris nevi are relatively common and require no treatment, malignant melanomas have the potential to metastasize and often require treatment. Ultrasonography plays an important role when differentiating between these diagnoses, but AS-OCT can also reveal the location, size and extent of a mass’s boundaries.
 |
|
Fig. 3. Limbal microcysts under a scleral contact lens exhibiting limbal vault (left). Corneal scarring and opacification identifying the corneal layers involved (right). Click image to enlarge. |
Limitations
While AS-OCT is an important tool for many clinicians, it’s important to note some limitations that exist. The operator must be familiar with the specific device they are using, as scan protocols and instrumentation differ amongst different manufacturers. For example, the Cirrus 6000 (Zeiss) device has a separate cornea and an angle attachment and its own proprietary software to expand the capabilities of its posterior segment device. The Anterion (Heidelberg) is the newest, dedicated AS-OCT device and unique in that it combines tomography and anterior segment imaging in one instrument.
To capture a successful image, the operator must be familiar with the purpose of the image and its proper acquisition techniques. For instance, when acquiring images of the anterior chamber angle during a glaucoma evaluation, the operator should take into consideration the lighting conditions, which affect pupil size. As pupil size changes, the angle either narrows or widens, making it difficult to compare images over multiple visits.16,17
Unlike posterior segment OCT, which has built-in progression analysis, AS-OCT has a lack of tracking between visits. Once the lighting conditions are determined, the patient must be appropriately positioned. Images that are not acquired perpendicular to the ocular surface run the risk of leading to a diffraction phenomenon, giving rise to distorted images.16 Common types of imaging artifacts include an inability to identify Schwalbe’s line or anterior iris surface, poorly aligned scan or poor penetrance when there is opacification. Even after controlling for the factors mentioned above, the quality of the image can be difficult to assess, as certain AS-OCT devices do not come equipped with an objective scoring system like signal strength quality found in posterior segment OCT.19 The operator, therefore, is left to subjectively determine the quality of the images.
After the image is found to be of good quality, different examiners may have their own interpretation of the position of the landmarks that they are looking for. This is another source of error that can lead to issues when comparing images over multiple visits or amongst different practitioners.19,20 Previous work has shown only a moderate level of agreement in grading AS-OCT photos.21 It is also important to mention that in the case of the anterior chamber angle evaluation, the light rays of the AS-OCT cannot penetrate the iris pigment epithelium; thus, the ciliary sulcus and posterior ciliary body cannot be imaged.22 This can greatly impact interpretation of the scans.
Finally, no matter what the purpose of the image is, there are measurement differences between different AS-OCT imaging software. For instance, a practitioner who wants to compare central corneal thickness between other AS-OCT devices will not be able to do so, as it will differ.
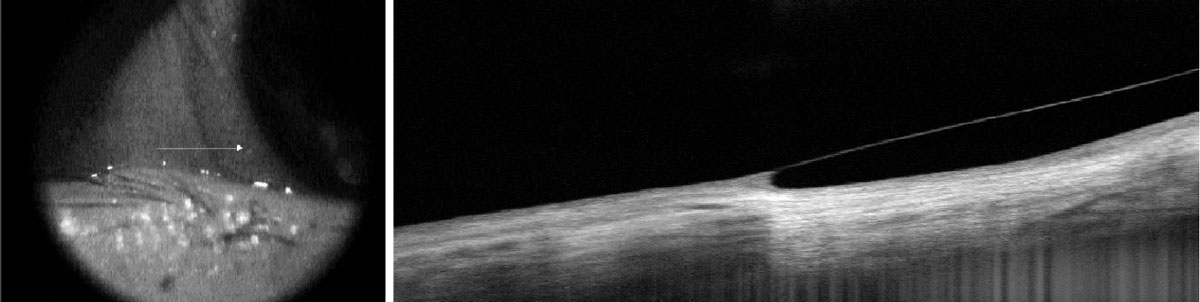 |
Fig. 4. Good scleral alignment of a scleral lens without impingement or lift-off. Click image to enlarge. |
AS-OCT vs. Corneal Tomography
When it comes to corneal shape, thickness and power, corneal tomography provides a great deal of information. Like AS-OCT, it captures cross-sectional images and provides deeper three-dimensional understanding of the cornea and its underlying angle. The Pentacam (Oculus) and AS-OCT have displayed good agreement in corneal thickness and anterior chamber depth measurements. Most AS-OCT devices do not have topography or tomography capabilities, with the Anterion being a notable exception.
AS-OCT vs. Gonioscopy
This eye test is frequently used to evaluate and monitor the angle of glaucoma patients and narrow angle suspects. One disadvantage of gonioscopy compared to AS-OCT is that it requires a skilled practitioner to perform and interpret the clinical appearance of the angle. The gonioscopy lens also contacts the patient’s cornea and requires patient cooperation. However, AS-OCT does not provide a dynamic view of the angle as gonioscopy cannot yet image neovascularization, pigment and/or peripheral anterior synechiae. Similarly, the superior and inferior quadrants are difficult to scan with AS-OCT without manipulating the angle configuration as only gonioscopy scan. Both procedures are useful to understand angle anatomy iris approach with AS-OCT preferred for patients unable to tolerate gonioscopy.
AS-OCT vs. Ultrasound Biomicroscopy (UBM)
This ultrasound technique is a useful tool to image much of the anterior segment anatomy, specifically by providing visualization of ciliary body and structures posterior to the iris, which AS-OCT does not perform as well.
Compared with AS-OCT, this technology has several limitations. The UBM probe contacts the ocular surface and requires patient cooperation and a skilled examiner. UBM also produces lower resolution images than AS-OCT. However, UBM is preferred when imaging certain conditions such as iris cysts and melanomas because it is better able to penetrate the iris pigment epithelium.
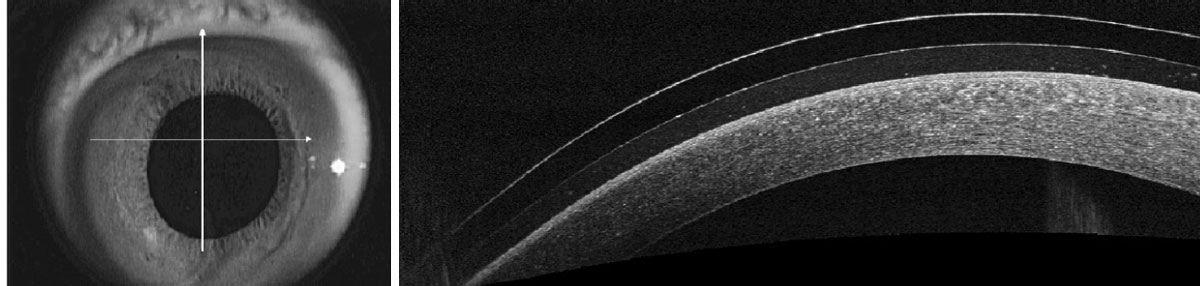 |
Fig. 5. Murky, debris-filled tear reservoir under a scleral lens. Click image to enlarge. |
Takeaways
There is no doubt that AS-OCT is a powerful imaging tool that allows us to measure, visualize and image cross-sectionally the anterior segment from tear film to lens. It is an amazing patient education tool when describing or illustrating the depth of a corneal scar or teaching patients about their iridocorneal anatomy. It is well-tolerated by patients, as it is non-contact and image acquisition times are short. Practitioners agree that training operators is straightforward, and that the interpretation of images is easier as the basic structures are the same as those visualized with slit lamp.
Given AS-OCT’s unique advantages and the possibility of future visibility of anterior segment vasculature, growing in popularity much like its posterior segment counterpart is highly anticipated.
Dr. Keh received her doctor of optometry degree from the Nova Southeastern College of Optometry and completed a cornea and contact lens residency at the SUNY College of Optometry. She has worked as an associate optometrist in a specialty contact lens-focused private practice in Manhattan and is currently an associate clinical professor at the SUNY College of Optometry. Dr. Keh is a fellow of the American Academy of Optometry and has presented and lectured at past Global Specialty Lens Symposium and American Academy of Optometry meetings. Dr. Frantzis completed both her doctor of optometry degree and cornea and contact lens residency at SUNY Optometry and is an assistant clinical professor at the SUNY College of Optometry. Yana Seviaryn is currently pursing her doctor of optometry degree at the SUNY College of Optometry and is completing a microcredential in cornea and contact lenses. They have no financial disclosures.
1. Ang M, Baskaran M, Werkmeister R, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132-56. 2. Huang AS, Belghith A, Dastiridou A, et al. Automated circumferential construction of first-order aqueous humor outflow pathways using spectral-domain optical coherence tomography. J Biomed Opt. 2017;22(6):066010. 3. Pang CE, Vanathi M, Tan DT, Mehta JS. Evaluation of corneal epithelial healing under contact lens with spectral-domain anterior segment optical coherence tomography (SD-OCT). Open Ophthalmol J. 2011;5:51-4. 4. Nanji A, Sayyad F, Galor A, et al. High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology. Ocul Surf. 2015; 13: 226-35. 5. Thomas, BJ, Galar A, Nanji AA, et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12(1):46-58. 6. Konstantopoulos A, Kuo J, Anderson A, Hossain P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am J Ophthalmol. 2008;146(4):534-42. 7. Steinberg J, Casagrande MK, Frings A, et al. Screening for subclinical keratoconus using swept-source fourier domain anterior segment optical coherence tomography. Cornea. 2015;34(11):1413-9. 8. Fuentes E, Sandali O, El Sanharawi M, et al. Anatomic predictive factors of acute corneal hydrops in keratoconus: an optical coherence tomography study. Ophthalmology. 2015;122(8):1653-9. 9. Sorbara L, Maram J, Fonn D, et al. Metrics of the normal cornea: anterior segment imaging with the Visante OCT. Clin Exp Optometry. 2010;93(3):150-6. 10. van der Worp E, Lampa M, Kinoshita B, et al. Variation in sag values in daily disposable, reusable and toric soft contact lenses. Cont Lens Anterior Eye. 2021;44(6):101386. 11. Itoi M, Itoi M, Harigaya A, et al. Corneal RGP contact lens fitting software for keratoconus built-in anterior segment optical coherence tomography. Eye Contact Lens. 2022;48(12):503-8 12. Yeung D, Luigina S. Scleral lens clearance assessment with biomicroscopy and anterior segment optical coherence tomography. Optom Vis Sci. 2018;95(1):13-20. 13. Koh V, Keshtkaran MR, Hernstadt D, et al. Predicting the outcome of laser peripheral iridotomy for primary angle closure suspect eyes using anterior segment optical coherence tomography. Acta Ophthalmol. 2019;97(1):57-63. 14. Jiang Y, Chang DS, Haogang Z, et al. Longitudinal changes of angle configuration in primary angle-closure suspects: the Zhongshan Angle-Closure Prevention Trial. Ophthalmology. 2014;121(9):1699-705. 15. Wang BS, Naranyanaswamy A, Amersinghe N, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95(1):46-50. 16. Han SB, Liu YC, Noriega KM, Mehta JS. Applications of anterior segment optical coherence tomography in cornea and ocular surface diseases. J Ophthalmol. 2016:4971572. 17. Shi Y, Marion KM, Jenkins D, et al. Identification and characterization of imaging technique errors and artifacts using anterior segment OCT for irido-corneal angle evaluations in glaucoma. Ophthalmol Glaucoma. 2019;2(3):136-44. 18. Zhang XF, Li M, Shi Y, et al. Repeatability and agreement of two anterior segment OCT in myopic patients before implantable collamer lenses implantation. Int J Ophthalmol. 2020;13(4):625-31. 19. Müller M, Dahmen G, Pörksen E, et al. Anterior chamber angle measurement with optical coherence tomography: intraobserver and interobserver variability. J Cataract Refract Surg. 2006;32(11):1803-8. 20. Salim S. The role of anterior segment optical coherence tomography in glaucoma. J Ophthalmol. 2012:476801. 21. Chansangpetch S, Nguyen A, Mora M, et al. Agreement of anterior segment parameters obtained from swept-souce fourier-domain and time-domain anterior segment optical coherence tomography. Invest Opthalmol Vis Sci. 2018;59(3):1554-61. 22. Li X, Zhou Y, Young CA, et al. Comparison of a new anterior segment optical coherence tomography and Oculus Pentacam for measurement of anterior chamber depth and corneal thickness. Ann Transl Med. 2020;8(14):857. |


