OCT: Eye Care’s All-Purpose ToolThis versatile device has dozens of functions in eye care today. In the January 2023 issue of Review of Optometry, our experts explore its capabilities in full. Check out the other articles featured in this OCT-themed issue:
|
Optical coherence tomography (OCT) is a valuable tool in optometric practice that plays a key role in various aspects of care, including managing posterior segment disease, glaucoma and even some anterior segment conditions. However, as technology evolves and the number of available options continues to grow, determining the best device to invest in comes with a host of considerations.
“When first deciding how to proceed with purchasing an OCT, a good place to start is to consider your patient population and what you want to achieve,” notes Jessica Haynes, OD, of the Charles Retina Institute in Memphis. “For example, some may want to integrate OCT imaging into their practice primarily as a screening tool, referring patients to a specialist when abnormalities are detected.
“Others may want to diagnose and manage a larger range of disease and send patients to specialists only when they require surgical interventions not within the optometrist’s scope of practice,” she continues. “These two physicians may have very different ideas about what instrument is necessary for their practices.”
“What specialty are you in or what clinical interest would you like to explore?” asks Michael Cymbor, OD, a partner at Nittany Eye Associates in State College, PA. “Are you interested in retina (age-related macular degeneration and diabetic retinopathy), optic nerve, ganglion cell and angle (glaucoma) or cornea (keratoconus scleral fits and refractive surgery)? Each manufacturer has strengths and weaknesses—you want to choose the company that matches well with you and your interests.”
Whether an OD is preparing to invest in their first OCT or is ready to upgrade their current device, it is important they have a clear picture of their needs and how the available options on the market can support their clinical practice. We spoke with several experts to gain their insights on best practices as well as challenges and missteps to avoid.
 |
|
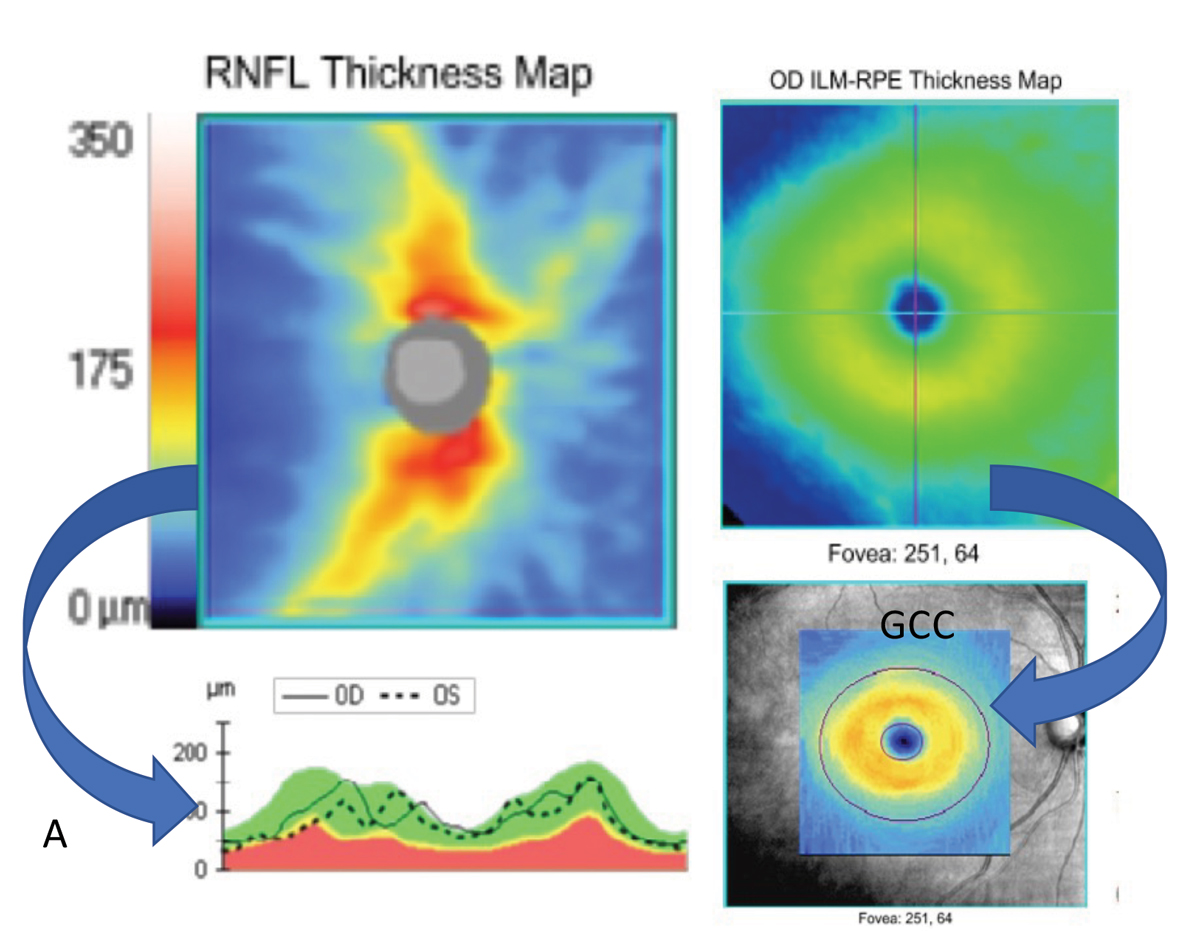
Fig. 1. Basic scanning patterns such as the optic disc cube (left) and macular cube (right) can be used to extrapolate a wealth of information such as RNFL thickness maps (top left) and RNFL TSNIT curves (bottom left), macular thickness maps (top right) and ganglion cell complex thickness maps (bottom right). These types of scans may also provide deviation plots to compare your patient to a normative database. Scans from a Zeiss Cirrus. Photo: Jessica Haynes, OD. Click image to enlarge. |
Tech Considerations
Since the first commercial OCT device came on the market in 1996, the technology has evolved exponentially. This has opened the door to a plethora of new uses; however, it has also made deciding which option is best for your clinical needs more complex.
Today, optometrists can use OCT to image a variety of structures in the eye, according to Dr. Haynes, who notes that OCT is classically thought about in terms of scans of the macula or scans of the optic nerve, with the typical macula scan being a “cube scan centered on the macula and the optic nerve scan showing the temporal superior nasal inferior temporal (TSNIT) or NITSN curve measurement of the retinal nerve fiber layer (RNFL).”
While these scan types are an important component of patient care, many devices can now go beyond basic scanning patterns. “They offer complex or even customizable scanning patterns of the macula, optic nerve, mid-peripheral and even peripheral retina,” explains Dr. Haynes (Figures 1 and 2).
Scan resolution is another important consideration. “Some instruments offer more densely spaced scans with lower resolution alongside higher resolution raster type scans,” says Dr. Haynes. “It is important to consider the resolution of scanning patterns that you will be using frequently (Figure 3).”
 |
|
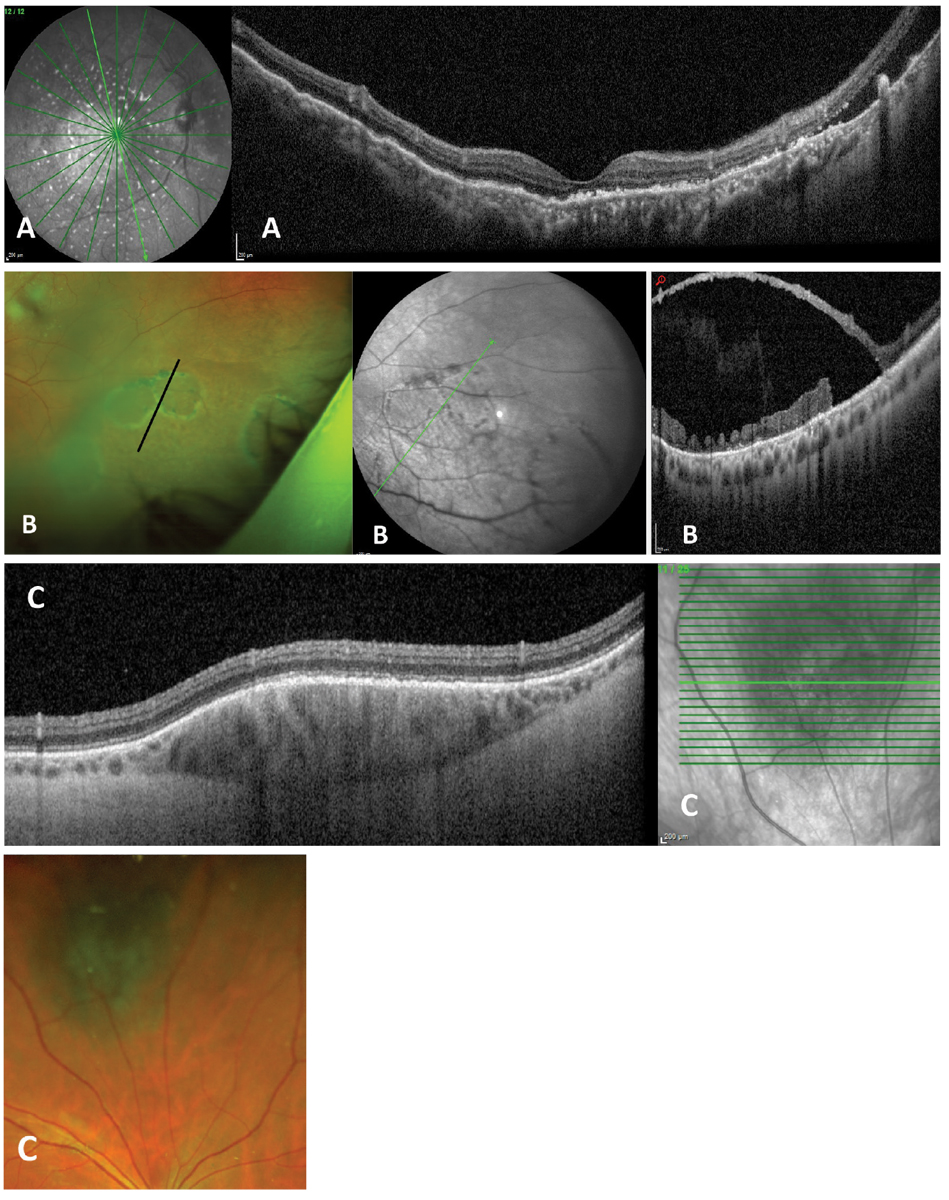
Fig. 2. Complex or customizable scanning patterns along with wider fields of view are available on some instruments. Examples are (A) 55° widefield radial scan through the macula of patient with uveal effusion syndrome. (B) Peripheral OCT of patient with large outer layer retinal breaks within a retinoschisis. (C) A customized cube scan through a midperipherally located choroidal nevus. All images from a Heidelberg Spectralis. Photo: Jessica Haynes, OD. Click image to enlarge. |
Spectral domain (SD) vs. swept source (SS). A key decision when selecting a device is whether you prefer SD-OCT or SS-OCT. While both offer high resolution retinal images, there are differences to consider.
“When evaluating different technologies, prioritize which features are most important to you and what best aligns with your practice’s needs,” says Julie Rodman, OD, a professor at Nova Southeastern University. “Current SD-OCT models provide outstanding resolution with a rapid acquisition time, capturing 26,000 to 80,000 axial scans per second.”
SS-OCT operates at a speed of 100,000 to 200,000 axial scans per second, allowing for more precise imaging of the deeper retinal layers and choroid, she explains, noting that the faster scan time with SS-OCT also allows for wider B-scans to assist with widefield images.
The superiority of SS-OCT in imaging deeper structures such as the choroid can be impactful in patients with pachychoroidal disease or when identifying and more clearly defining the extent of an occult choroidal neovascular membrane on OCT angiography (OCT-A), according to Dr. Haynes. “How this may impact use in every day clinic would depend on the individual provider and which types of disease they want to manage.”
She also noted that some SD instruments offer enhanced-depth imaging (EDI)—a scanning strategy that can be selected for patients with suspected pathology that affects deeper tissue such as buried optic nerve head drusen. For optometrists purchasing an SD-OCT, Dr. Haynes recommends asking if the device comes with the EDI option.
So, how do you decide which option is best for your clinical practice? While every individual practice is unique and has its own specific needs, Danica Marrelli, OD, assistant dean of clinical education at the University of Houston College of Optometry, says that a SD-OCT without angiography is going to be sufficient for the vast majority of optometrists. However, that’s not to say this is the case for everyone or that there isn’t value in investing in newer technology when possible.
“There is an argument for purchasing the very latest technology you can afford because technology is only going to continue to move forward,” Dr. Marrelli suggests. “And I do believe we are moving more and more towards SS-OCT. That being said, you’re not going to go wrong with a good SD-OCT.”
 |
|
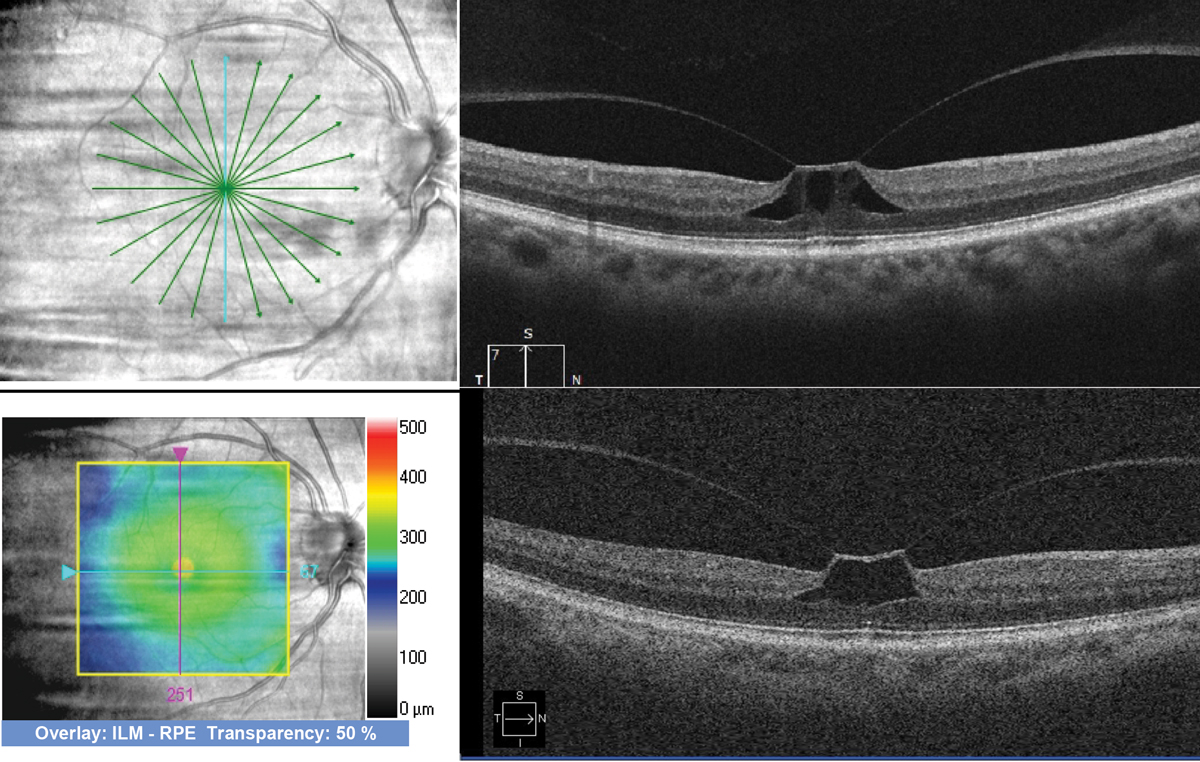
Fig. 3. Comparison of a high-resolution radial scan (top) and lower-resolution cube scan (bottom) performed on a patient with vitreomacular traction. Both are good quality scans, but they are different types, with the radial scan offering higher resolution and more slices through the fovea. Scans taken with Zeiss Cirrus OCT. Photo: Jessica Haynes, OD. Click image to enlarge. |
OCT-A. There are several technologies on the market that offer OCT-A—a non-invasive imaging modality that provides visualization of the retinal and choroidal vasculature. “When used alongside OCT, OCT-A rounds out the complete picture of structure and vascular integrity,” notes Dr. Rodman.
“This technology is particularly useful in medically oriented practices, where common pathologies, including diabetic retinopathy and age-related macular retinopathy, are seen routinely,” she says. “Capillary nonperfusion, microaneurysms, retinal ischemia and choroidal neovascularization can be seen without the use of intravenous dye.”
Dr. Marrelli, who works in a glaucoma and retina heavy practice, has found that angiography is an interesting tool that, at this point, is not something they typically use to make clinical decisions. “It creates some really nice images, but it doesn’t direct our management at this point that much more than an SD-OCT,” she notes.
“Certainly in the glaucoma area, it’s interesting to look at the angio of the blood vessels surrounding the optic nerve, but we really don’t know what to do with it yet,” she explains. “From a clinical standpoint, I don’t know that we fully understand how to use the information we can gain from this tool yet in glaucoma patients. I think we’ll get there, but I don’t know if we are there right now.”
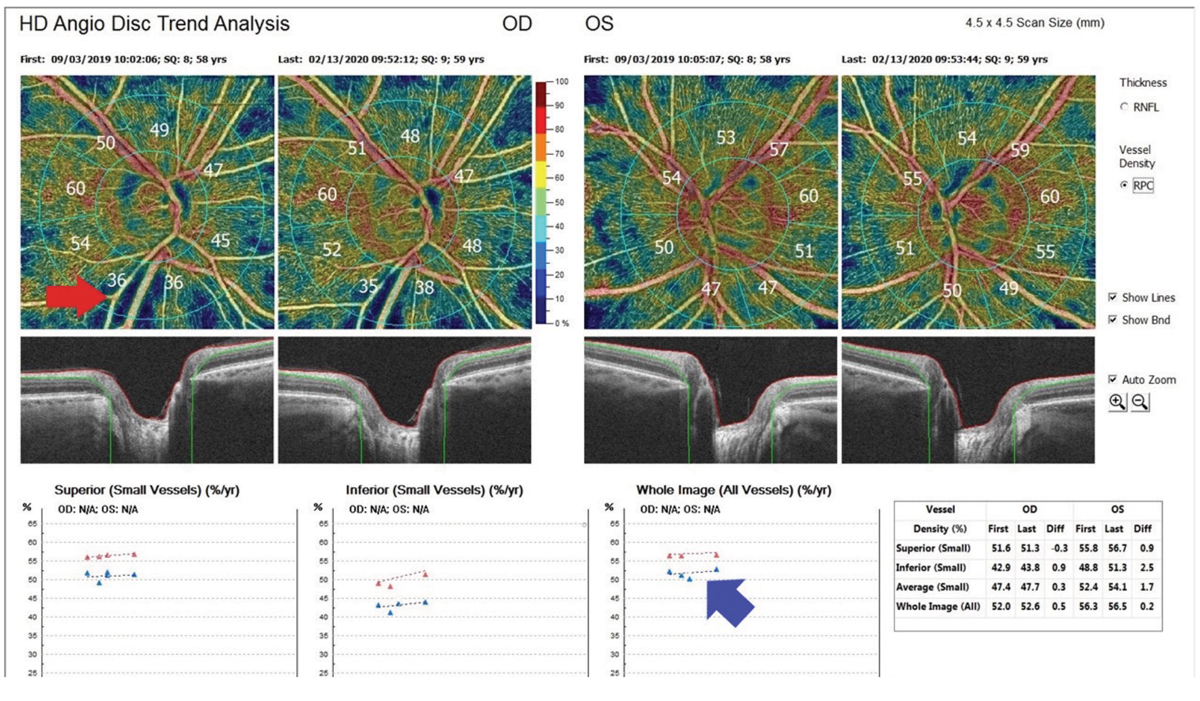
Other clinicians do find this capability to be useful in glaucoma today. Dr. Cymbor says that “OCT-A is helpful to me in all stages of the glaucoma spectrum. The technology not only allows me to see RNFL defects easier, it helps me determine treatment efficacy. I would find it difficult to run our glaucoma clinic without OCT-A.” (Figure 4)
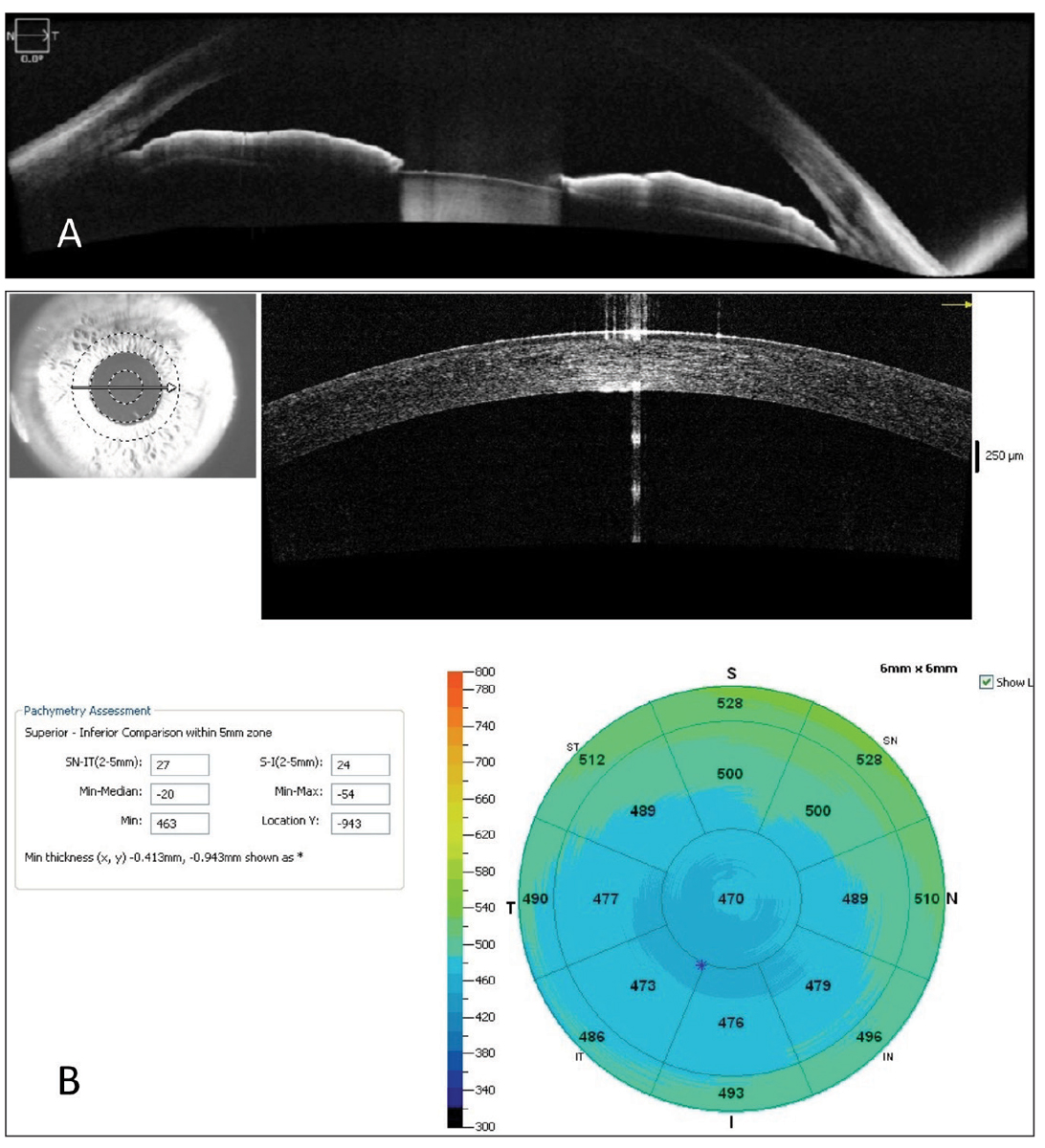
Anterior segment. Most OCTs can also scan the anterior segment. This can be used to evaluate iris and angle anatomy in patients in the narrow angle spectrum, evaluate contact lens clearance when fitting scleral lenses, evaluate the penetrating thickness of a corneal foreign body and obtain optical pachymetry measurements, according to Dr. Haynes, who notes that consideration should be given if these are available with a particular instrument (Figure 5).
There are now some anterior-specific devices available that can offer improved imaging of this area than the instruments that are engineered for posterior segment use but have since added an anterior segment module to the software, says Sharon Keh, OD, assistant professor at SUNY College of Optometry. “Dedicated anterior segment OCT devices use deeper, higher wavelengths allowing for better imaging and resolution of the cornea and other anterior segment structures,” she notes, while adding that most practices opt for posterior segment devices, which are more mainstream and have a longer list of billable diagnoses.
 |
|
Fig. 4. This is an image of OCT-A glaucoma progression analysis from an Avanti from Visionix (Optovue). The top left red arrow shows a nerve fiber layer defect, and the bottom right blue arrow shows optic nerve perfusion improvement after treatment. Photo: Michael Cymbor, OD. Click image to enlarge. |
While a dedicated anterior segment OCT is probably not the right choice for most ODs, there are some that may find this option a good fit for their practice, Dr. Keh says. “Most practices who have this device are those with a very high volume of specialty contact lens fittings or OD/MD clinics that comanage complex corneal disease and glaucoma,” she notes. “For the most part, anterior segment OCT allows for more precise imaging but has yet to replace an in-depth clinical evaluation, e.g., gonioscopy.”
“Some OCTs, such as our Heidelberg, have anterior-segment OCT capabilities and even fundus photo and fluorescein angiogram options but require that separate special lenses be attached to the machine in order to capture these,” says Sara Weidmayer, OD, of the Kettles Medical Center VA in Ann Arbor, MI. “In our busy practice, whose OCT is in use non-stop all day—overwhelmingly for posterior segment OCTs—these lens swaps are actually fairly cumbersome, so it is prohibitive” when used at high volume.
“As scan speeds improve, it is only a matter of time until we have devices that are able to effectively image all layers of the eye from eyelid to cornea,” notes Dr. Cymbor.
Viewing and analytic software. While the raw reflectivity data of OCT is often viewed as a cross-section OCT B-scan, explains Dr. Haynes, the instruments allow for visualization of the data in a variety of ways, including en face OCT imaging and thickness maps, among others. Thickness maps may involve the ability to view full retinal thickness or even that of individual retinal layers.
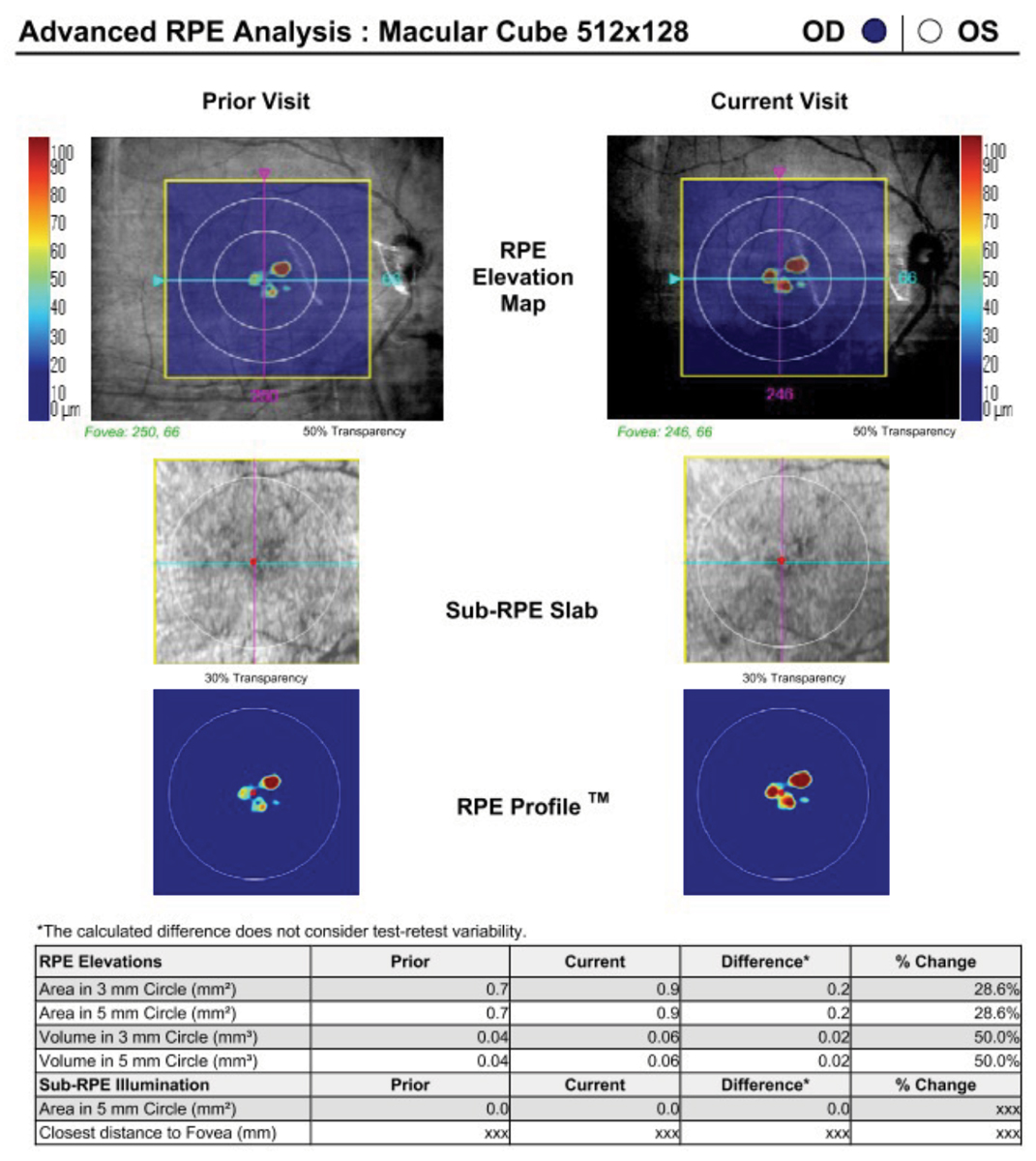
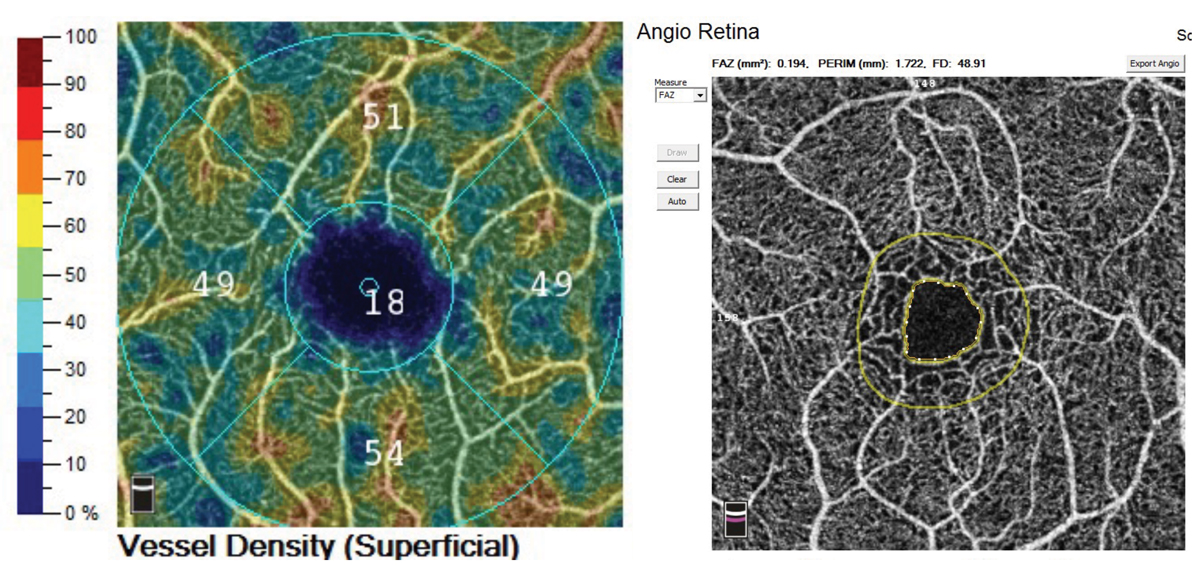
“Instruments are unique in the software analysis that they provide,” Dr. Haynes notes. “For example, the Cirrus by Zeiss has a program that allows the visualization of drusen volume that can be tracked for change over time (Figure 6). Another example would be that while many instruments offer OCT-A, the Optovue Angiovue from Visionix provides a software package called AngioAnalytics that gives numerical data about the size of the foveal avascular zone and vascular density (Figure 7).”
 |
|
Fig. 5. An anterior segment OCT (A) used to evaluate a patient with anatomically narrow angles taken with Zeiss’s Cirrus OCT, and (B) optical pachymetry measurements performed with the Optovue Avanti OCT from Visionix. Photo: Jessica Haynes, OD. Click image to enlarge. |
Also consider tracking software, which is particularly valuable in glaucoma as well as retina care, where the ability to detect structural change over time is critical, according to Dr. Haynes. Some questions to ask include:
- How does the software allow the physician to look for change over time?
- Can progression analysis be viewed efficiently in a busy practice?
- What technology is used to ensure that scans are accurate and repeatable over time?
When making buying decisions, it can also be helpful to think about how you can view your OCT information, recommends Dr. Haynes. “Can you add a desktop viewer to each work station? Or will you have to rely on viewing a pre-selected printout sent to your EMR in your exam room?”
Dr. Weidmayer’s VA clinic initially had a license for only so many viewing stations, which she says was terribly inconvenient for workflow, since they had many more doctors than available viewing stations. “This created a ruckus of having to track down doctors to log off so others could log on,” she explains. “Especially for the retinal scans and OCT-A, the pre-selected printouts or reports are not adequate for decision-making, so the workstation viewing software—or using the machine itself—is critical.”
“While many optometrists are fine with reviewing standard OCT reports, some will prefer using viewing software to access each scan slice to pick up pathologies like subtle paramacular drusen that is easily missed in current reports,” adds Dr. Cymbor.
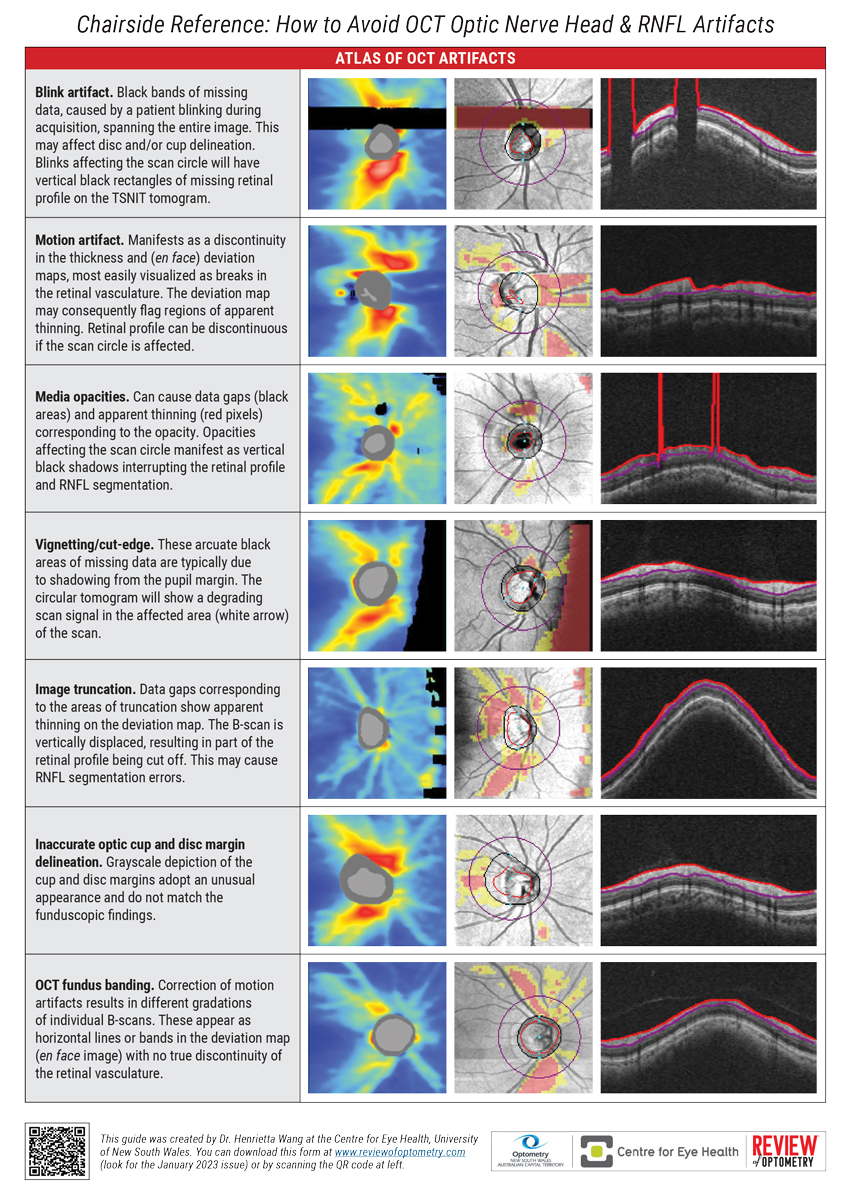
Overcoming OCT ArtifactsBy Henrietta Wang, BOptom (Hons), BSC, MPHThe guiding principle in all of healthcare is primum non nocere, or “first, do no harm.” While we usually think of that in a treatment context, this can also be applied to the diagnostic techniques we employ on a day-to-day basis. If the device itself introduces errors, this can undermine the clinical care we provide by confounding accurate diagnosis. OCT technology is extremely precise but not infallible. Artifacts—faulty data arising from one or more errors in scan acquisition—are surprisingly common in OCT use. They can arise from one of four sources: (1) the patient: small pupils, media opacities, poor fixation or anomalous retinal planes (2) the instrument: segmentation errors, inadequate depth of view, limited refractive range or posterior shadowing (3) the operator: alignment or centration error, inappropriate scan selection or focusing mistakes (4) the disease: the presence of concomitant pathology (e.g., ERM or PPA) Our team at the Centre for Eye Health (UNSW) has developed a set of guidelines that appears on the next two pages. They can help you minimize the confounding effects of OCT artifacts. Feel free to photocopy or download it for use in your practice. Best of luck! |
Value of Other Features
Many of the platforms available today offer a variety of multimodal imaging capabilities that go beyond the traditional OCT experience. This includes fundus autofluorescence (FAF), OCT-A, fluorescein angiography, indocyanine green angiography, fundus photography and multispectrum imaging. But how important are they for the optometrist? As with other buying decisions, this will depend on a practice’s individual needs.
“It is extremely beneficial to have the capability of visualizing a fundus image simultaneously with the OCT scan. This way, pathology can easily be located on the fundus image and cross-referenced with the OCT B-scan,” notes Dr. Rodman. “As previously mentioned, clinics that are seeing a high volume of medical retina would certainly benefit from the addition of OCT-A. This technology allows for pre-emptive diagnosis of disease in turn optimizing care of our patients.”
Dr. Cymbor adds, “There is a substantial number of diabetes patients whose only structural change is diabetic macular ischemia. This ischemia can only be found on OCT-A or fluorescein angiography. Early diagnosis allows us to recommend more aggressive care at a more appropriate time point, leading to better patient outcomes.”
 |
|
Fig. 6. A macular cube scan on the Cirrus can be used to extrapolate and track drusen volume using its Advanced RPE Analysis software. Photo: Jessica Haynes ,OD. Click image to enlarge. |
The ability to view the retina with multiple imaging modalities can provide a more complete picture of disease, improving diagnostic and management capabilities, says Dr. Haynes. This may also, she notes, allow the instrument to be used for a wider patient range and billing codes.
“However, it is important to keep in mind that there are not currently separate CPT codes for each of these imaging strategies. For example, both OCT and OCT-A of the macula would use CPT code 92134 and both FAF and fundus photography would use CPT code 92250,” advises Dr. Haynes. “While this is frustrating for physicians from a billing standpoint, the ability to properly and independently manage a wider variety of disease can ultimately create increased revenue for the practice, improve patient outcomes and increase job satisfaction.”
In addition to the potential clinical benefits of being able to image the patient in numerous ways, devices with multiple modalities can be a good option when space is an issue.
“If an optometrist needs to minimize the footprint of their instruments, combination devices that offer fundus photography and OCT can be a good option,” says Dr. Marrelli.
“Be mindful that some units that combine OCT and fundus photography offer poorer resolution than stand-alone camera systems,” says Dr. Cymbor.
 |
| Chairside Reference: How to Avoid OCT Optic Nerve Head & RNFL Artifacts Guide by Henrietta Wang, BOptom (Hons), BSC, MPH. Click image to enlarge. |
 |
|
|
Tech Support and Office Integration
Prior to purchasing an OCT, optometrists must take the time to time to have an in-depth discussion with the manufacturer of the device(s) they are considering. Don’t hesitate to ask questions and raise any concerns you may have.
One of the most important questions to ask, according to Dr. Rodman, is what kind of support/service they will receive after the sale. “Support may come in the form of website access to informational portals or direct communication with a liaison that is familiar with the technology,” she notes.
Understanding the level of support that will be available is critical, not just in terms of the technology itself, but also when incorporating these devices into clinical practice, notes Dr. Haynes. Several key questions to consider include:
- How will the instrument be maintained?
- How will it integrate into the EMR?
- Who is going to train your staff on how to use the instrument?
- What happens if there is a problem with the software or the hardware?
“Having a highly trained, easily accessible technical support team is crucial as these problems will undoubtedly come up,” says Dr. Haynes, while advising ODs to inquire about training services, technical support and warranty plans before making a purchase.
 |
|
Fig. 7. The Optovue Angiovue OCT-A platform offers AngioAnalytics, a software package that computes vessel density and foveal avascular zone size that can be tracked over time. Photo: Jessica Haynes, OD. Click image to enlarge. |
Dr. Cymbor notes, “Sometimes these items are negotiable, so don’t miss an opportunity to put yourself in a more attractive position.”
Dr. Marrelli reiterates the importance of training and technical support. “This is something I feel very strongly about,” she says. “Someone should come to your office, and they need to train you and your staff until you feel comfortable with the instrument.”
This support should go beyond using the device, Dr. Marrelli explains. “Optometrists should have support not only with how to use the device and get the best quality scan but also how to really understand the report and analyze the images,” she says. “Both types of training are important and need to be included in your purchase price.”
This is also, in Dr. Marrelli’s opinion, one of the drawbacks of buying a used OCT. “Buying an instrument from someone who, for instance, is looking to upgrade, may get you a great deal financially, but are you going to have the support and training you need? Training is critical for optimizing the impact the device has on your clinical practice.”
Another key consideration is upgrade paths. If you want to initially purchase an OCT-only device right now, would you have the option to upgrade to obtain other platforms like FAF or OCT-A in the future?
It is critical that ODs have a clear understanding of the upgrades—and warranty—associated with the specific device they purchase, according to Dr. Marrelli. Are you going to have to pay for every software upgrade? Will you have to replace instruments? Will the upgrades be downloadable and easy to install? Or will someone have to come to your office for the installation?
“It is important ODs know what they are signing up for,” she emphasizes. “Are you going to have to pay for every new feature or new upgrade? This can get expensive, so optometrists must keep this in mind in terms of their budget.”
Another consideration is maintenance and service agreements, which can be very costly, Dr. Marrelli adds. “A smaller practice, or a practice that has a lower budget, may want to prioritize these considerations. An instrument that is going to be under warranty for a longer period of time and includes upgrades can be a very appealing option.”
 |
|
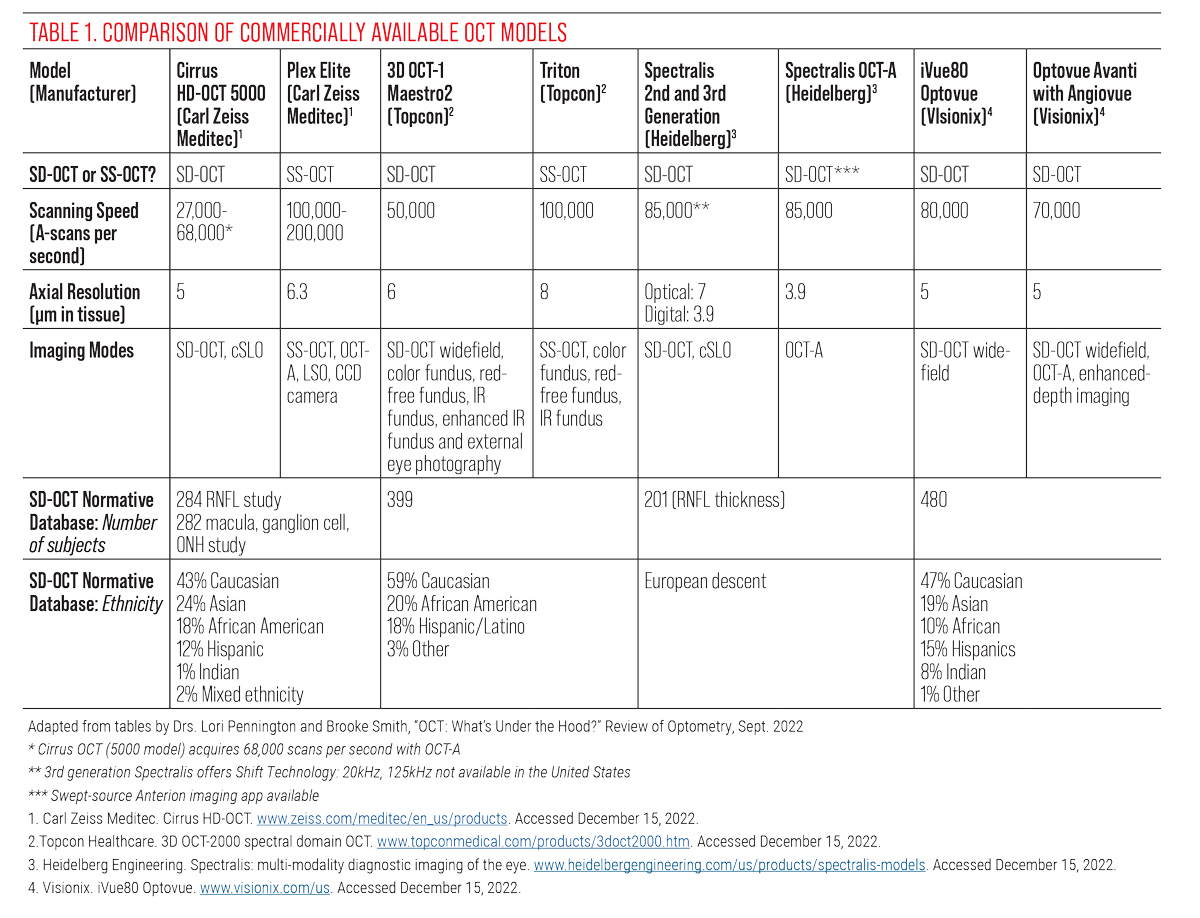
Table 1. Comparison of Commercially Available OCT Models. Adapted from tables by Drs. Lori Pennington and Brooke Smith. Click image to enlarge. |
Potential Challenges and Missteps
Don’t fall into the trap of thinking that all instruments do the same thing, urges Dr. Haynes. “There is such a variety of OCT instruments on the market,” she says. “They all have their pros and cons, but they don’t all do the same things. You really have to consider your patient population and clinic needs and find a device that best fits that need.”
The second biggest mistake, in Dr. Haynes’s opinion, is not using your OCT instrument to its fullest extent. If an OD has really done their research into the device they are purchasing, they should already be well versed in its capabilities.
“But in the event that you end up with an OCT instrument that you are not familiar with, be sure to take the time to learn all you can about the instrument,” Dr. Haynes notes. “It may have scanning patterns, viewing software, progression analysis and other tools that enhance your ability to detect, accurately diagnose, monitor and manage disease, if only you learn how to use them.”
A key issue ODs will have to contend with is staff training and avoiding scan accuracy issues. “In most situations, the doctor will not be the person obtaining scans,” notes Dr. Haynes. “Does your practice have a highly trained ocular photographer or a skilled technician? If so, you may not have to worry about ease of use. If not, you may have to consider an instrument that is more ‘point and click.’”
“This also goes hand-in-hand with support provided by the manufacturer,” says Dr. Rodman. “It is very important that the doctor inquires about training. How is it done? Is there access to videos? Is there a liaison that could answer questions, if needed?”
Billing is also an important component. “For instance, a patient comes in for a vision exam, and you find something that needs to be medically evaluated. In that case, you need to separate that visit, re-appointment them and then do it under medical,” says Dr. Marrelli. “So, optometrists who buy OCTs need to be on medical plans, they need to understand how to navigate vision versus medical and learn how to accurately bill.”
Another question ODs may have is whether or not they need to stay with one brand for all of their devices. Is there a platform “lock-in” effect where you feel like you need to buy the Zeiss instrument because you have other Zeiss devices and years of patient data on their platform? According to Dr. Rodman, the answer is no.
“Most companies have platforms whereby you can merge different technologies and view on a common portal,” she explains. “Oftentimes, these companies partner with other companies who can provide this service to their customers, so they don’t feel locked into a particular brand.”
However, an OD upgrading their existing OCT may find it easier to opt for the same brand when purchasing their new device, notes Dr. Marrelli.
“We’re at a time now where instrument companies are pretty sensitive to the fact that we need to be able to have backward compatibility with former technology. However, to my knowledge, we don’t really have anything that would allow you to, for example, transfer 10 years of data from a Zeiss instrument to a Topcon instrument,” she says.
“When you change brands, you’re not going to be able to directly compare and so there is some value in staying with the same brand in terms of bringing that data forward into the new instrument.”
Staying with the same company does not always assure the use of previous patient data. Dr Cymbor says, “Sometimes new OCTs are unable to use previous data from older devices even within the same company, so you might start over for things like glaucoma progression analysis.”
Ultimately, the optometrist must make their own choice and own that decision. The best way to do that is to be as informed as possible.
“Ask the vendor if they will let you try out the unit in your office before purchasing. Using the device and ‘playing’ with it in your setting is the most important thing to do,” says Dr. Rodman.
“This will allow the doctor to assess the ease of use and the frequency of use of the various modalities offered on the device,” she concludes. “What’s good for one practice may not be for another. The needs of one practice may not match the needs of another.”


