 For several years now, corneal collagen crosslinking (CXL) has received periodic attention in the ophthalmic literature and press. First described in 1998 and reported as a viable clinical procedure in 2003, CXL is a minimally invasive surgical technique that utilizes a photoactivating solution (riboflavin) in combination with UV-A light energy to induce crosslinking of collagen fibrils. This process serves to increase corneal tensile strength and rigidity.1,2
For several years now, corneal collagen crosslinking (CXL) has received periodic attention in the ophthalmic literature and press. First described in 1998 and reported as a viable clinical procedure in 2003, CXL is a minimally invasive surgical technique that utilizes a photoactivating solution (riboflavin) in combination with UV-A light energy to induce crosslinking of collagen fibrils. This process serves to increase corneal tensile strength and rigidity.1,2
Recently, our colleagues Paul Karpecki, O.D., and Diana Shechtman, O.D., published an excellent overview of CXL in their “Research Review” column (see “
A Look at Corneal Crosslinking,” August 2011), which described the utility of this procedure in the management of keratoconus and post-LASIK ectasia.
And while CXL has been shown to strengthen the cornea and arrest or reverse progressive corneal ectasias, there have been recent reports of other applications for CXL. Most notably, this technique has shown promise as an adjunctive therapy in the management of microbial keratitis.3
Antimicrobial Effect of CXL
Early reports of the potential efficacy of CXL against ocular pathogens were first published in 2008, when Suy Anne R. Martins, M.D., Ph.D., and associates documented successful results using riboflavin/UV-A in vitro to destroy bacterial isolates of Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pneumoniae grown in agar plates.3 Their work showed limited overall susceptibility for Pseudomonas aeruginosa, and in the case of Candida albicans (a fungal pathogen), there was no measurable effect.3 Still, the authors postulated that in vivo CXL might prove to be a valuable adjunct to pharmaceutical management for severe or recalcitrant cases of bacterial keratitis.
In 2009, Hans Peter Iseli, M.D., and associates presented a series of five patients who successfully underwent CXL to treat infectious corneal melts that were refractory to conventional antimicrobial pharmacotherapy.4 Interestingly, the majority of these patients had developed these infections following LASIK surgery, and the recovered microbes included such atypical organisms as Mycobacterium and Fusarium.
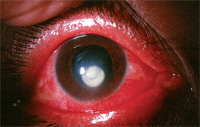
Someday, clinicians may use corneal collagen crosslinking as a primary therapy for bacterial keratitis, as seen in this patient.
Dr. Iseli and his colleagues suggested two possible mechanisms for the positive outcomes achieved. First, they noted that the CXL process induced greatly enhanced collagen resistance to digestive enzymes, such as those produced by bacteria and fungi in infectious keratitis.4,5 Additionally, they noted that directed UV-A can inhibit the growth of bacteria and fungi, and the oxygen-free radicals produced during the CXL procedure were believed to interfere with microbial cell wall synthesis and repair.6,7
In subsequent years, numerous reports detailed the success of CXL in the treatment of recalcitrant infectious keratitis.8-12 One case series evaluated seven eyes with severe corneal ulcers and progressive melting, despite the use of topical antibiotic therapy in five of the patients.11 CXL treatment was performed (using the standard protocol for keratoconus) and arrested corneal melting and permitted complete re-epithelialization in 100% of subjects. In all but one eye, patients showed symptomatic improvement within 24 hours following the procedure.
Most recently, a pilot study investigated the use of CXL as a primary therapy for bacterial keratitis.13 This prospective, non-randomized clinical trial followed 16 patients through ocular photography, microbial culturing and treatment with CXL. Isolated bacterial strains from this study included Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus species, Corynebacterium, Propionibacterium and Pseudomonas aeruginosa.13
As per the protocol, subjects did not receive topical antibiotics unless they exhibited signs of infectious progression. Following CXL treatment, all 16 eyes exhibited diminished symptoms and decreased inflammation. The use of concurrent antibiotic therapy was unnecessary in 87% of eyes (14 of 16 eyes). Epithelial healing was achieved in 100% of cases.13
Research scientists have also investigated the use of CXL for treatment of more uncommon and severe corneal infections, such as Acanthamoeba and fungal keratitis. In vitro studies have shown less than favorable results for these pathogens, and a recent in vivo animal study of CXL for experimental Acanthamoeba keratitis was unable to demonstrate any significant clinical efficacy.14-16
CXL for Keratoconus
While treatment of infectious keratitis with CXL is still considered experimental, acceptance of the procedure for treating keratoconus is growing among practitioners.
In September 2011, the FDA granted Avedro, Inc. orphan drug designation for its VibeX (0.1.% riboflavin) solution to use with its KXL System. In FDA terms, “orphan status” allows a company to develop new therapies for a rare disease or medical disorder that affects fewer than 200,000 individuals in the United States.17 More importantly, it permits the company to sell its product for seven years––without competition––as well as obtain clinical trial tax incentives.
The Corneal Collagen Cross-linking for Progressive Keratoconus (CXL) Study has been ongoing since 2008 at multiple clinical sites throughout the country.18 Reports in several trade journals have suggested that the procedure may receive full FDA approval before the end of 2012, although this is purely speculative.
To date, just one report from this study has been published.18 In a single cohort, Peter Hersh, M.D., and associates reported overall improvement in both uncorrected and corrected visual acuity, maximum K value and average K value within one year after a single CXL treatment. Patients in the control group showed no such improvement in any of these parameters.
New technologies continue to enhance our ability to care for patients with visual disorders and ocular diseases. Optometrists must remain current with procedures, such as CXL, if we are to remain competitive in the ever-changing world of ocular therapeutics.
Drs. Kabat and Sowka are consultants for Alcon Laboratories. They have no direct financial interest in any of the products mentioned.
1. Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998 Jan;66(1):97-103.
2. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003 May;135(5):620-7.
3. Martins SAR, Combs JC, Noguera G, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: A potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3402-8.
4. Iseli HP, Thiel MA, Hafezi F, et al. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008 Jun;27(5):590-4.
5. Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007 May;26(4):385-9.
6. Yoshimura M, Namura S, Akamatsu H, et al. Antimicrobial effects of phototherapy and photochemotherapy in vivo and in vitro. Br J Dermatol. 1996 Oct;135(4):528-32.
7. Jeong J, Kim JY, Yoon J. The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ Sci Technol. 2006 Oct 1;40(19):6117-22.
8. Micelli-Ferrari T, Leozappa M, Lorusso M, et al. Escherichia coli keratitis treated with ultraviolet A/riboflavin corneal cross-linking: a case report. Eur J Ophthalmol. 2009 Mar-Apr;19(2):295-7.
9. Ehlers N, Hjortdal J, Nielsen K, Søndergaard A. Riboflavin-UVA treatment in the management of edema and nonhealing ulcers of the cornea. J Refract Surg. 2009 Sep;25(9):S803-6.
10. Morén H, Malmsjö M, Mortensen J, Ohrström A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea. 2010 Jan;29(1):102-4.
11. Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010 Dec;29(12):1353-8.
12. Anwar HM, El-Danasoury AM, Hashem AN. Corneal collagen crosslinking in the treatment of infectious keratitis. Clin Ophthalmol. 2011;5:1277-80. Epub 2011 Sep 7.
13. Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2011 Aug 27. [Epub ahead of print]
14. Del Buey MA, Cristóbal JA, Casas P, et al. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet a for Acanthamoeba isolates. Am J Ophthalmol. 2011 Oct 11. [Epub ahead of print]
15. Sauer A, Letscher-Bru V, Speeg-Schatz C, et al. In vitro efficacy of antifungal treatment using riboflavin/UV-A (365 nm) combination and amphotericin B. Invest Ophthalmol Vis Sci. 2010 Aug;51(8):3950-3.
16. Kashiwabuchi RT, Carvalho FR, Khan YA, et al. Assessing efficacy of combined riboflavin and uva light (365 nm) treatment of Acanthamoeba trophozoites. Invest Ophthalmol Vis Sci. 2011 Nov 4. [Epub ahead of print]
17. FDA U.S. Food and Drug Administration. Developing Products for Rare Diseases & Conditions. Available at: www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/default.htm (accessed November 14, 2011).
18. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011 Jan;37(1):149-60.

