A 64-year-old black female with a longstanding history of glaucoma was well overdue for her progress evaluation. When the patient did finally return, she had several new complaints as well as a major change in her medical history.

She reported that she had been evaluated for cracked ribs of an unknown etiology. Subsequent X-rays led to further evaluation and an unexpected diagnosis. A lifelong smoker, she had lung cancer. Prior to today’s exam, she had undergone surgery to remove a major part of her right lung lobe and was awaiting chemotherapy.
Today, she reported several changes. She said that her right eyelid had become droopy. Also, she had weakness in her right hand as well as significant subscapular discomfort radiating beneath her armpit. She couldn’t remember if these changes had begun before lung surgery or after, but she believed things had worsened greatly immediately after surgery.
She had asked her other treating physicians about these findings, but they discounted a relationship with her cancer diagnosis.
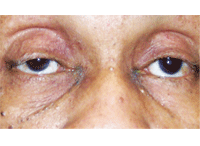
New onset right ptosis (with miosis) in a patient who presented with Horner’s
syndrome secondary to a Pancoast tumor.
Upon examination, a pronounced ptosis was seen in her right eye that had never been present before. Additionally, her pupils measured 2.5mm O.D. and 3.5mm O.S. in full illumination, and 3.0mm O.D. and 6.0mm O.S. in dim illumination with a dilation lag. Previously, she was documented as being isocoric.
This all pointed to a dysfunction within the sympathetic nervous system, namely the oculosympathetic plexus. Clearly, she had a new onset of Horner’s syndrome. Combined with her history of lung cancer and lobectomy as well as her other physical symptoms, it was evident that she had Pancoast syndrome precipitated by lung cancer. In this month’s column, we will discuss Horner’s syndrome.
What is Horner’s Syndrome?
Horner’s syndrome is characterized by an interruption of the oculosympathetic nerve supply somewhere between its origin in the hypothalamus and its termination in the eye.1 The classic findings associated with Horner’s syndrome are ptosis, pupillary miosis and facial anhydrosis.
Sympathetic innervation to the eye involves a continuous pathway consisting of three neurons. The first neuron (considered a central neuron) originates in the dorsolateral hypothalamus, descending through the brain stem and traveling to the ciliospinal center of Budge, between the levels of the eighth cervical and fourth thoracic vertebrae (C8-T4) of the spinal cord.
It then synapses with the second neuron (which is considered pre-ganglionic) whose cell bodies give rise to axons, which exit the white rami communicantes of the spinal cord via the anterior horn. These axons pass over the apex of the lung and enter the sympathetic chain in the neck, synapsing in the superior cervical ganglion.
At this point, the third neuron gives rise to post-ganglionic axons that course to the eye to form the long and short posterior ciliary nerves. These sympathetic nerve fibers course anteriorly through the uveal tract and join the fibers of long posterior ciliary nerves to innervate the dilator of the iris. Sympathetic fibers also innervate the muscle of Müller, responsible for initiating eyelid retraction during eyelid opening. Damage at any location along this pathway (central, pre-ganglionic or post-ganglionic) will induce an ipsilateral Horner’s syndrome.
Testing for Horner’s Syndrome
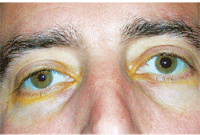
Another patient with a recent onset of Horner’s syndrome O.S. following Iopidine (apraclonidine, Alcon) testing. Miosis and ptosis on the left side was reversed 30 minutes after Iopidine instillation.
The diagnosis and localization of Horner’s syndrome can be accomplished with pharmacological testing.2 In this dysfunction, there is a lack of the sympathetic neurotransmitter, norepinephrine. The iris dilator does not receive sympathetic stimulation in Horner’s syndrome, thus accounting for the miosis that increases in dim light conditions and the dilation lag (relative to the normal contralateral pupil) when lighting is reduced.
Cocaine is a sympathomimetic drug which acts indirectly to prevent reuptake of endogenously released norepinephrine from the sympathetic nerve terminal. A topically applied dilution of 10% cocaine produces pupillary dilation in the normally innervated pupil by blocking the reuptake of norepinephrine at the nerve ending. The Horner’s pupil will dilate poorly compared to the normal eye because of the sympathetic dysfunctional absence of norepinephrine at the nerve ending. So, either poor or no dilation with cocaine is indicative of Horner’s syndrome.
Topically applied cocaine identifies Horner’s syndrome, but does not indicate where the problem lies within the sympathetic three-neuron arc. To identify the lesion as being central or pre-ganglionic (neurons 1 or 2 before the synapse in the superior cervical ganglion) or post-ganglionic (after exiting the superior cervical ganglion), Paredrine hydroxyamphetamine 1% (Paredrine hydroxyamphetamine 1%, Akorn Pharmaceuticals) can be subsequently used 72 hours later (after the effects of the topical cocaine have dissipated). Paredrine acts by forcing the release of endogenous norepinephrine from the presynaptic vesicles. If the third neuron is damaged, the pupil will not dilate because there is no norepinephrine to release, indicating a post-ganglionic lesion. If the Horner’s pupil does dilate upon Paredrine instillation, the third neuron is healthy, and the damage involves either the first or second neuron; this condition is considered a pre-ganglionic Horner’s syndrome. Currently, there is no method of topical testing to differentiate a first order pre-ganglionic lesion from a second order pre-ganglionic lesion.
Unfortunately, these drugs (cocaine and hydroxyamphetamine) are not available for clinical practice; however, it appears that the intraocular pressure lowering drug apraclonidine (0.5% and 1% Iopidine, Alcon) is a viable option. Apraclonidine is an alpha-2 adrenergic agonist that seems to also stimulate alpha-1 receptors to a small degree. Pupil dilation in suspected cases of Horner’s syndrome is considered diagnostic.
The theory behind this is that the Horner’s syndrome pupil undergoes denervation hypersensitivity. When a very weak alpha-1 adrenergic agonist is applied, the hypersensitive pupil dilates while the normal pupil has no effect.3-6 In most cases, there will actually be a reversal of the anisocoria, which is easier to appreciate than the asymmetric dilation induced by cocaine. It appears that the most readily available agent, Iopidine 0.5%, is at least as sensitive and specific in the diagnosis of Horner’s syndrome as cocaine.6
What is Pancoast Syndrome?
Pancoast syndrome is a malignancy of the superior pulmonary sulcus causing subsequent destruction of the thoracic inlet and involvement of the brachial and oculosympathetic plexuses.7 Most cases involve non-small cell lung carcinoma. As the oculosympathetic plexus courses over the superior aspect of the lung, it is prone to compression by a malignant space-occupying lesion. This would cause a second order lesion. Horner’s syndrome is accompanied by shoulder pain radiating to the axilla and scapula. There is also atrophy of the hand and arm with resultant muscle weakness. Bony structures of the chest are often invaded by the malignancy, especially the thoracic vertebrae and ribs.
Based upon the clinical picture of a new onset ptosis and ipsilateral miosis with dilation lag, the patient was diagnosed with Horner’s syndrome. Additional pharmacologic testing was considered unnecessary at this point. Due to her recent diagnosis of lung cancer with surgical removal, rib involvement, scapular pain and hand weakness, it was clear that the Horner’s syndrome was a manifestation of Pancoast syndrome.
Further evaluation was also deemed unnecessary. She was educated that all of her recent ocular complaints were indeed related to the cancer and surgery. At this time, the patient is undergoing cancer treatment.
1. Wilkins RH, Brody IA. Horner’s syndrome. Arch Neurol. 1968 Nov;19(5):540-2.
2. Maloney WF, Younge BR, Moyer NJ. Evaluation of the causes and accuracy of pharmacologic localization in Horner’s syndrome. Am J Ophthalmol. 1980 Sep;90(3):394-402.
3. Mughal M, Longmuir R. Current pharmacologic testing for Horner syndrome. Curr Neurol Neurosci Rep. 2009 Sep;9(5):384-9.
4. Morales J, Brown SM, Abdul-Rahim AS, Crosson CE. Ocular effects of apraclonidine in Horner syndrome. Arch Ophthalmol. 2000 Jul;118(7):951-4.
5. Bacal DA, Levy SR. The use of apraclonidine in the diagnosis of Horner syndrome in pediatric patients. Arch Ophthalmol. 2004 Feb;122(2):276-9.
6. Koc F, Kavuncu S, Kansu T, et al. The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. Br J Ophthalmol. 2005 Nov;89(11):1442-4.
7. Sartori F, Rea F, Calabro F, et al. Carcinoma of the superior pulmonary sulcus. J Thorac Cardiovasc Surg. 1992 Sep;104(3):679-83.

