 |
A 68-year-old African American female presented to the clinic for a routine eye examination with a chief complaint of blurred vision in the right eye of six months’ duration.
Her ocular history was remarkable for slightly enlarged cup-to-disc ratios and previously identified but untreated cataracts OU. Discussion of her systemic history revealed no reports of hypertension, diabetes or other illnesses. The patient denied any past ocular trauma or allergies to medications or environmental substances.
Clinical Findings
Her best uncorrected entering visual acuities were 20/150 OD and 20/25 OS at distance and near with no improvement upon pinhole or refraction. Her external exam was normal with the exception of the facial Amsler grid OD, which demonstrated central distortion. There was no afferent pupillary defect.
Biomicroscopy uncovered normal anterior segment tissues with grade II nuclear sclerotic cataracts in both eyes. Her intraocular pressures measured 16mm Hg by Goldmann applanation. The pertinent posterior segment findings are demonstrated in the photographs.
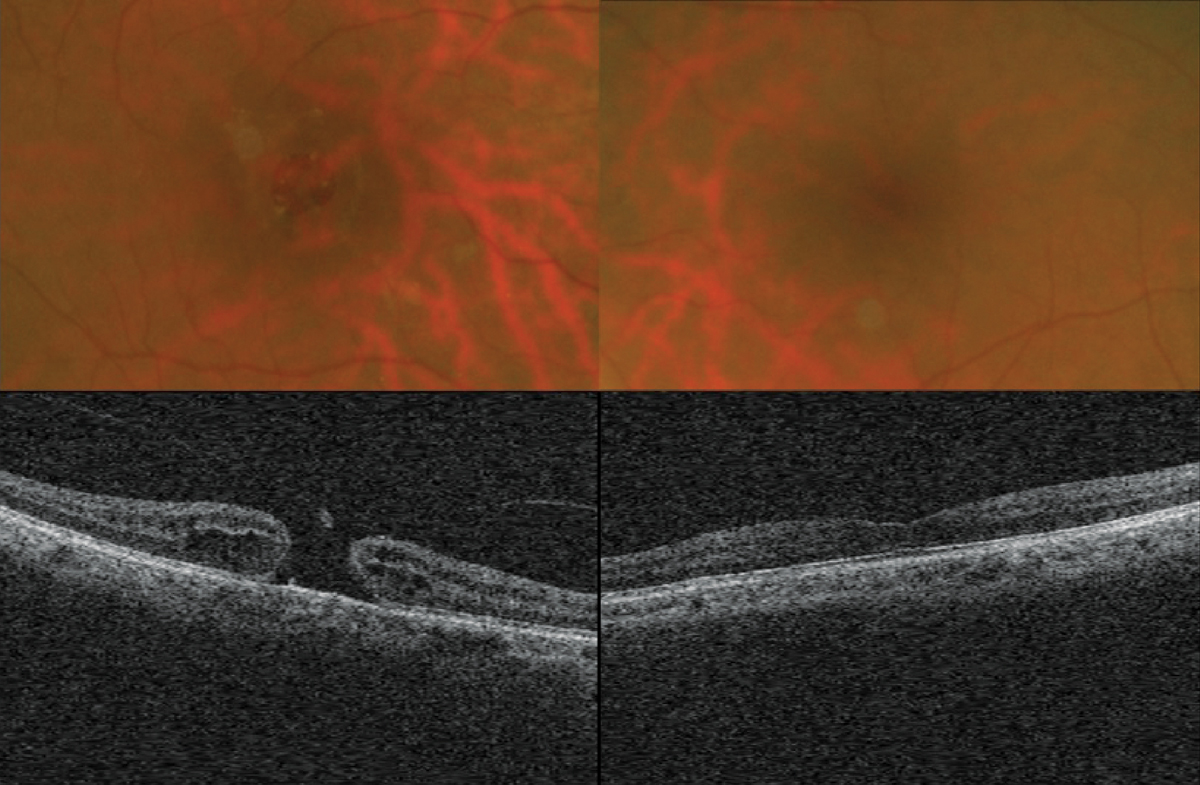 |
|
While the retinal findings above should be fairly clear, it is appropriate to consider whether or not the patient’s circumstances add additional complexity to the management of the case. Read the online discussion of this case for a thorough assessment of options. Click image to enlarge. |
For Additional Information
Additional testing included a traditional Amsler grid, formal automated perimetry, additional funduscopic examination with a 90-diopter lens completing the Watzke-Allen sign (a vertical strip of light perceived by the patient as “broken” or distorted), OCT testing and laser interferometry to assess best acuity.
What would be your diagnosis in this case? What is the patient’s likely prognosis?
Diagnosis
This patient is experiencing a full-thickness macular hole OD in the setting of vitreomacular traction OD with vitreomacular adhesion OS. The differential diagnosis for macular hole includes four clinical entities: lamellar macular hole (LMH), epiretinal membrane (ERM)/vitreomacular adhesion and traction (VMA/VMT) and macular pseudohole (MPH).1-6
Macular holes are defined as anatomic areas of neurosensory retinal discontinuity within the fovea.1-4 The most accepted mechanism for the pathogenesis of macular holes is pathologic vitreoretinal traction at the vitreoretinal interface (VRI).1-4 Macular holes may range from the incipient stages (macular cyst with or without serous foveal detachment), resulting in only minor visual disturbances (metamorphopsia), to full-thickness lesions with catastrophic endpoints.1-6
While the epidemiology of macular holes may vary depending on the diversity of the population, as a rule, some characteristics are universally accepted: (1) macular holes occur more frequently in people over the age of 75, (2) they occur more frequently in women than men at all ages and (3) they are typically unilateral.2-6
Visual symptoms vary depending upon the hole’s location, staging and severity, ranging from normal visual function (20/20) without symptoms to mild metamorphopsia to visual loss correlating to the size location and injury to the macular tissue.1-8
Pathophysiology
The majority of macular holes are idiopathic in nature; this is especially true in older patients (6th-7th decade of life).1,3,4 The prevalence of macular holes is thought to be 3.3 to 8.7 per 100,000 persons.3-5 Other associated causes of macular hole include trauma, solar retinopathy, degenerative/pathologic myopia and intravitreal triamcinolone used in the management of other vitreoretinal events. 3-6
The vitreous plays an important role in the pathophysiology of macular hole formation.1-8 The vitreous supports the structure of the eyeball. It consists of collagen (mostly type II) that runs anteroposteriorly.3,4,6-9 The space between the collagen fibrils is filled with a gel that is composed of hyaluronic acid, the protein opticin, the fibril-associated glycosaminoglycan chondroitin and water (98%).3,4,6-9 The strongest adhesions between the vitreous and the retina (known as the vitreoretinal interface) occurs along the vitreous base, the optic disc, the area around the fovea or area centralis and along all major blood vessels.4,6-9 In general, the adhesion between vitreous and retina is nested in the internal limiting membrane of the neurosensory retina and is accomplished through the interactions of the extracellular matrix.3,9,10 The main constituents of this system include proteoglycans, laminin and fibronectin.3,4,9,10
With age (starting at approximately around 40 years), two processes contribute to vitreous degradation: vitreous syneresis (gel aggregation) and synchysis (gel liquefaction).3,4,6-9 Both of these work together to create the formation of intravitreal fluid-filled pockets (lacunae), which have the ability to enlarge, coalesce and migrate.3,4,6-9 Over time, these pockets can grow in size and number, and even come together to weaken vitreous adhesions to the internal limiting membrane.3,4,6-10 As the process occurs, collagen fibrils collapse and coalesce.3,4,6-10 These remnants float inside the liquid pockets and create the entoptic phenomenon known as “floaters” when they cast moving shadows across the retina.4,11,12
When these pockets of gel move about during flicks, drifts, saccades, glissades or head movement, they exert tangential tractional forces on the neurosensory retina, which creates mechanical phosphenes. This produces the entoptic phenomenon of “flashes.”4,11,12
In unfortunate circumstances, where the retina is thin, holes or tears can develop, permitting liquid vitreous to seep under the edge of the break and create a rhegmatogenous retinal detachment.4,12
During any stage of this evolving process, the pockets of liquified vitreous can involve the VRI and cause it to deconstruct.3,4,6-10 As the vitreous detaches from the retina, it creates what is known as an incomplete posterior vitreous detachment (PVD). This can be found in up to 50% of the general population entering the third decade of life.4,6-10 A complete PVD (where the vitreous has detached from the optic disc) is seen in up to 50% of all 70-year-olds.3,4,7,8 Here, the complete or incomplete remnant of this circular attachment can be visualized floating in the vitreous and is known as a Weiss ring.2,3,4,6,7,9
A perifoveal incomplete PVD can complicate the relationship of the VRI through persistent vitreomacular adhesion.3,4 The persistent adhesions can interrupt the internal limiting membrane, creating incongruities through which retinal glial cells, retinal fibrous astrocytes, Müller cells, myofibroblasts, macrophages and RPE cells can migrate.3,4,8-10 These, along with the remnants of degenerated vitreous collagen, create the scaffolding known as epiretinal membrane (ERM) formation.3,4,8-10 It is proposed that myofibroblast contraction leads to ERM thickening and puckering, as well as retinal holes/detachments.3,4,8-10
Classically, macular holes were classified in 1988 by Gass, who noted there were four stages based on fundus examination.2-4,6,13,14 In 2013, the International Vitreomacular Traction Study Group (IVTS) updated the Gass classification with the use of OCT imaging.3,13 They created an OCT-based anatomic classification system for diseases of the vitreomacular interface.3,13 This group created anatomic definitions and classifications of VMA, VMT and macular hole.3,13
VMA is a perifoveal vitreous detachment that is characterized by an elevation of the cortical vitreous above the retinal surface with the remaining vitreous attached within a 3mm radius of the fovea.3,4 This can be further subclassified as focal (1500µm) or broad (>1500µm).3,4 VMA is largely asymptomatic.3,4,13
As any incomplete PVD progresses it can lead to traction that causes anatomic changes, which can be identified with OCT—once identified, this is termed VMT.3,4 These structural changes are described as distortions of the foveal surface, intraretinal structural changes, elevation of the fovea about the RPE or any combination of these.3,4 VMT is also further subclassified as focal (1500µm) or broad (>1500µm).3
The anatomic changes in VMT often lead to symptoms such as metamorphopsia, photopsia, blurred vision and decreased visual acuity.3,4 When the changes are exaggerated, layers of the neurosensory retina become separated—this is termed foveal schisis.1.3 When the traction exceeds tissue tensile strength, it becomes discontinuous, forming a dehiscence, starting the process of hole formation.1,3,4
Macular Hole Staging Criteria
In his classic publications, Gass devised the standard for classifying and characterizing macular holes, laying the framework for the IVTS system.2-4 He separated the progression of macular holes into four stages:
Stage 1 (macular cyst) is defined by a serous detachment of the fovea. In early stage 1 holes (stage 1-A), the concavity of the fovea is lost and a yellow spot representing increased visibility of the xanthophyll pigment appears in the center of the macular area. Later (stage 1-B), the pigment is displaced outward, towards the circumference of the impending hole, causing it to appear ring-shaped.2-5,13 This change in pigment appearance from a spot to a ring is uniquely peculiar to macular hole development.2,4,5
Stage 2 (early macular hole) is defined by full-thickness retinal dehiscence. Biomicroscopically, it appears as an oval-, crescent- or horseshoe-shaped retinal defect on the inside edge of the xanthophyll ring. It can emerge as a central, round retinal defect surrounded by a rim of elevated retina with or without an overlying pre-foveal opacity. These macular holes may enlarge secondary to centrifugal movement of retinal receptors.18
Stage 3 full-thickness macular hole (FTMH) is defined by its size (400µm to 600µm diameter hole) and is usually surrounded by a rim of elevated retina. The vitreous becomes separated from the fovea and a pre-foveal opacity, representing this separation, may or may not be visible. PVD is not present. 2,4
Stage 4 macular holes are FTMHs that demonstrate detachment of the posterior vitreous.2,4 A pseudo-operculum, if present, is usually found near the temporal border of the Weiss ring.1,2,6,15
A classic FTMH appears as a symmetric, round, red lesion with a definable border secondary to the lifted and displaced foveal retinal tissue.2-7,12,13 The foveal defect includes all neural retinal layers from ILM to RPE.2-7,13 The IVTS subclassifies macular holes based on size, presence or absence of VMT and cause (primary or secondary).3 A primary FTMH results from VMT.3-6 A secondary FTMH is caused by other pathologies, such as trauma or high myopia.3-6
The size of a macular hole is based on its aperture size (measured with OCT); defined as small (up to 250µm), medium (250µm to 400µm) and large (>400µm).3
Some patients having FTMH also have VMA or VMT. Those fellow eyes can be classified as having “impending” macular hole.3 These individuals are at an increased risk of developing FTMH.3
It is important to distinguish between a full-thickness macular hole, a lamellar macular hole and a macular pseudohole.1-4,13-16
The latter entity resermbles a macular hole due to its reddish appearance at the fovea but, with an OCT an ERM, can be observed with a central opening, steep macular contour to the fovea and no loss of foveal tissue.3,13,15,16
A lamellar hole is a partial-thickness foveal defect that is best differentiated with an OCT due to the presence of an irregular foveal contour, absence of thickness defect, and presence of intact photoreceptors and intraretinal cysts.2,3,13-16 In both instances, the visual acuity and Amsler grid findings will be largely intact as the anatomy remains intact such that major symptoms or loss of function are avoided.
Prognosis and Intervention
The prognosis for recent-onset macular holes, not complicated by additional concurrent macular or ocular pathologies or caused by trauma, is good.3,4,13,17 The most important predictor of visual prognosis and anatomical hole closure is preoperative visual acuity.13,17 Various treatment options are available, including observation, vitreous injection and surgery.13-25
In small macular holes, there is a chance of spontaneous closure.13,18 In most cases, spontaneous closure of macular hole is seen in stages I-II. The rate for spontaneous closure not requiring intervention is 4% to 11.5%. This is typically a fortunate event seen in smaller diameter holes (<250µm).13,21-25
When traction is visualized and symptoms are present, consideration can be given for chemical vitreous release with ocriplasmin (Jetrea, Thrombogenics), the only non-surgical treatment for macular hole.22,24,25 Ocriplasmin is indicated for cases of symptomatic VMA with or without a FTMH.21-25 The compound is an enzyme that breaks down the retinal interface by activating matrix metalloproteinase-2.22,24,25 The Microplasmin for Intravitreous Injection-Traction Release without Surgical Treatment (MIVI-TRUST) studies demonstrated best closure rates in small macular holes (<200µm) with VMA.22,24,25 Resolution of vitreomacular adhesions was seen in 50% of patients with FTMH at day 28 post injection and FTMH closure occurred in 35% by month six.24,25 Macular holes at stage 2 or more do not benefit from Jetrea.22,24,25
The current gold standard treatment for idiopathic macular holes involves a three-port pars plana vitrectomy, ILM peel, gas tamponade and postoperative prone positioning.13,18 Postsurgical results depend on a variety of factors, including the technique used for ILM peel, the type of gas tamponade used and duration of prone positioning.13,18-21
The standard for prone positioning following most procedures is seven to 14 days. Recent studies have used swept-source OCT technology to permit the retina specialist to shorten the duration of prone positioning depending upon closure status.19,20 Generally, MH closure rate, after vitrectomy, is reported in the literature to be 86% to 97% with significant improvements in vision.17, 20, 22,23
The use of adjuvant cytokine therapy to promote hole closure seems to raise the prospect of complete anatomic closure. However, it has not shown improvement in postoperative acuity.18 Due to cost, complexity and complication rates, today’s macular hole procedures rarely indicate use.18
There are different techniques used for ILM peeling. In the preferred “inverted flap” technique, the ILM is peeled around the fovea but remains attached to the hole at the edges.13,22 The flap is then laid over the hole like a blanket or placed into the hole like a plug.12,22 The “inverted flap” acts as a barrier, preventing vitreous from entering the hole and as a scaffold, stimulating glial cell proliferation.13,22 Data suggests its use creates improved anatomical restoration of the fovea.13,22
Current research is examining new autologous platelet-rich plasma plugs to aid the ILM flap.13,22,23 The work has yielded some improved anatomical results and significant improvements in acuity.13,22,23
To aid ILM peeling, vital dyes such as indocyanine green (ICG), brilliant blue G and trypan blue may be used intraoperatively. These substances allow the surgeon to better visualize ILM, permitting safer and more complete removal. ICG use is controversial because some data suggests it may impose toxic effects on retinal pigmented epithelial cells.13,18,22 Of the three, brilliant blue has shown the best affinity for the ILM, allowing best visualization in the setting of less toxicity.13,22
Postsurgical results suggest prone patient recovery positioning (face down) is the best policy for patients with repairs of FTMH greater than 400µm.13,18 When expansile gas is used, prone positioning allows the bubble to migrate upward (vapor density <1), applying pressure against the macula that prevents the revised hole from reopening, permitting glial cell–mediated healing.13,18,19 Studies have shown equal efficacy in long term vs. short term posturing.13,17,19,20 An OCT-guided approach is being investigated to determine closure status as early as two hours after surgery.13,19,20 This new initiative may result in reduced need for inconvenient prone positioning.19,20
Macular holes can reopen after repair, with greatest risk in patients with myopic degeneration and chronic or large holes.13,19,20 Up to 10% of cases do not close, producing what is known as a “refractory FTMH.”13,18,22These are associated with greater size (<500µm), chronicity (longer than six months), high myopia, incomplete ILM peel and failed gas tamponade secondary to poor bubble maintenance or inability to keep prone positioning.13,18,22 Treatment for these cases requires repeating the vitrectomy, enlarging the ILM peel and maintaining strict postsurgical regimens.13,18,22
Encouraging Results
When referring to a retina specialist one can educate patients on treatment options and procedure protocols. When undergoing surgery, patients may be prescribed topical preparations including cycloplegics, antibiotics, antibiotic steroid preparations, ocular hypotensives and steroids as warranted.
This woman was referred to a retina specialist and underwent a successful procedure, resulting in restoration of 20/25 function. She was informed about the cause of the issue and was given an Amsler grid for home monitoring with instructions to return immediately in between visits if visual symptoms were noticed.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.

