 |
As awareness of meibomian gland disease (MGD) grows, so too have available treatments that directly address the unique biology of this common contributor to ocular surface disease. From supplements and lid hygiene to in-office debridement, blepharoexfoliaion and expression therapies, MGD treatment has evolved exponentially and continues still.
Intense pulsed-light (IPL) therapy is becoming an increasingly more mainstream alternative for patients with confirmed MGD, particularly if they also present with rosacea. Recently, a new form of IPL therapy has emerged using a two-step process that integrates traditional IPL with low-level light therapy (LLLT), a method of treating MGD using red to near-infrared light energy. This column reviews recent research into the modality.
 |
|
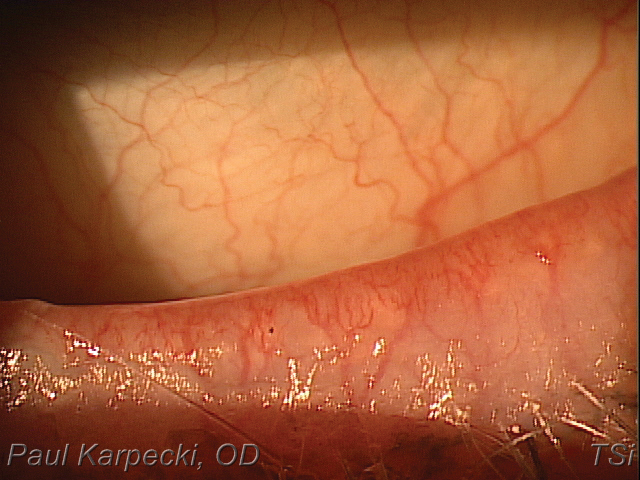
Telangiectasia with ocular rosacea may be responsive to treatment with combined IPL/LLLT. Click image to enlarge. |
What We Know About IPL
The value of IPL for the treatment of dry eye was first identified in 2002 by Rolando Toyos, MD, when patients who were being treated for skin problems reported improvements in their dry eye symptoms.1 This makes sense, as greater than 80% of rosacea patients have concomitant MGD.2
IPL treatments are performed with 500nm to 1,200nm light pulses for 20 to 30 minutes; it can be repeated every four to five weeks. Potential mechanisms whereby IPL can achieve clinical improvement include:3
- Thrombosis of abnormal or telangiectatic blood vessels below the skin surrounding the eyes.
- Heating the meibomian glands and liquefying the meibum.
- Activation of fibroblasts and enhancing the synthesis of new collagen fibers.
- Decreasing the bacterial and pathogen load on the eyelids.
- Interference with the inflammatory cycle by regulation of anti-inflammatory agents and MMPs.
- Reducing the turnover of skin epithelial cells and decreasing the risk of physical obstruction of the meibomian glands.
- Changes in the levels of reactive oxidative species.
IPL is generally considered safe; however, do not consider treatment in patients with Fitzpatrick skin type IV or lower, due to the risk of melanin damage and resultant hypopigmentation.4 Newer devices such as the Eye-Light (Innova Medical Ophthalmics) allow treatment for higher skin type ranges by adjusting the energy based on a measurement of skin pigment.
IPL Procedure BasicsAlthough there is some variation in protocol, standard IPL procedures involve placing protective eye shields over the eyes at the outset. Some systems require applying ultrasound gel on the skin to keep the treatment area cool. Treat only the skin inferior and lateral to the lower eyelid margin, as there is risk of light penetration through the eyelid and absorption within the intraocular structures with upper eyelid treatment.8 After two passes on each side, remove the ultrasound gel and apply a hot compress along the eyelids for two to three minutes.8 It is also been shown to be beneficial to express the meibomian glands following IPL treatment.9,10 In fact, meibum expressibility improvement might be a good therapeutic target of IPL treatment in patients with MGD and dry eye and could be an indicator of ocular surface inflammation during IPL treatment. In a recent study of 30 patients who underwent three IPL sessions, patients with low meibum expressibility and tear film instability experienced greater improvement in symptoms after IPL treatment.11 The improvement in meibum expressibility was also associated with a decrease in tear inflammatory cytokine levels.11 Finally, a topical steroid may be prescribed for two to three days following the procedure.8 |
Adding a Second Light Source
LLLT is a different form of photobiomodulation than IPL, but one that also began in dermatology and is now demonstrating efficacy in MGD, specifically in terms of improved tear break-up time.5,6
Combined light therapy involves the application of both IPL and LLLT.5
While IPL treatment offers thermal-based effects, LLLT is athermal and presumed to have additive photobiomodulation effects on the lids and periorbital area.5 The proposed mechanism of LLLT is photoactivation.5,7 The ability to apply LLLT to the upper lid, where it is generally considered unsafe to apply IPL, may further contribute to MGD improvement.5
A recent study of 460 eyes evaluated the effects of combined light therapy on patients who were unresponsive to previous medical management.5 The combined treatment consisted of intense, short pulses of light on the area of the face around the eye, followed by longer exposure to low-level red light on the cheek and over the closed lids.5
Researchers found that mean OSDI scores were significantly lower after combined treatment.5 Prior to treatment, 70.4% of patients had OSDI scores indicative of dry eye, whereas only 29.1% of patients had abnormal OSDI following treatment.5 A one-step or greater reduction in MGD grading was also observed in 70% of eyes, with 28% having a two-step or greater reduction.5 Tear break-up time was ≤6 seconds in 86.7% of eyes prior to treatment and dropped to 33.9% of eyes after treatment.5 There were no ocular or facial adverse events or side effects related to treatment.5
Beyond efficacy and safety, practical benefits may also inspire use of combined light therapy. Specifically, the EyeLight device adjusts energy levels for optimum effects based on the patient’s level of MGD and the Fitzpatrick skin scale score.5
Further, no gel is required, due to a built-in cooling system of forced air that maintains the temperature of the crystal at a non-traumatic level for the patient’s skin type.5
With so many tools at our disposal, we are well equipped to treat both the signs and symptoms of dry eye, ocular rosacea and MGD with greater ease and efficacy than ever before.
Dr. Karpecki is medical director for Keplr Vision and the Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
| 1. Kala. Patient survey; performed by a third party. 2. Ousler GW III, Abelson MB, Johnston PR, et al. Blink patterns and lid-contact times in dry eye and normal subjects. Clin Ophthalmol. 2014;8:869-74. 3. Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf. 2007;5(3):228-39. 4. Willcox MD, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366-403. 5. Dartt DA, McCarthy DM, Mercer HJ, et al. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Research. 1995;14(11):993-1000. 6. LeDoux MS, Zhou Q, Murphy RB, et al. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001;42:2434-41. 7. Van Der Werf F, Baljet B, Prins M, et al. Innervation of the lacrimal gland in the cynomolgous monkey: a retrograde tracing study. J Anat. 1996;188(Pt3):591-601. 9. Oyster Point Pharma announces positive results in ONSET-2 phase 3 trial of OC-01 nasal spray for the treatment of the signs and symptoms of dry eye disease. https://investors.oysterpointrx.com/news-releases/news-release-details/oyster-point-pharma-announces-positive-results-onset-2-phase-3. May 11, 2020. Accessed October 22, 2020. 8. Kala Pharmaceuticals announces statistically significant results in STRIDE 3 trial of dry eye disease drug Eysuvis. Eyewire. eyewire.news/articles/kala-pharmaceuticals-announces-statistically-significant-results-in-stride-3-trial-of-dry-eye-disease-drug-eysuvis. March 20, 2020. Accessed October 22, 2020. |

