 |
|
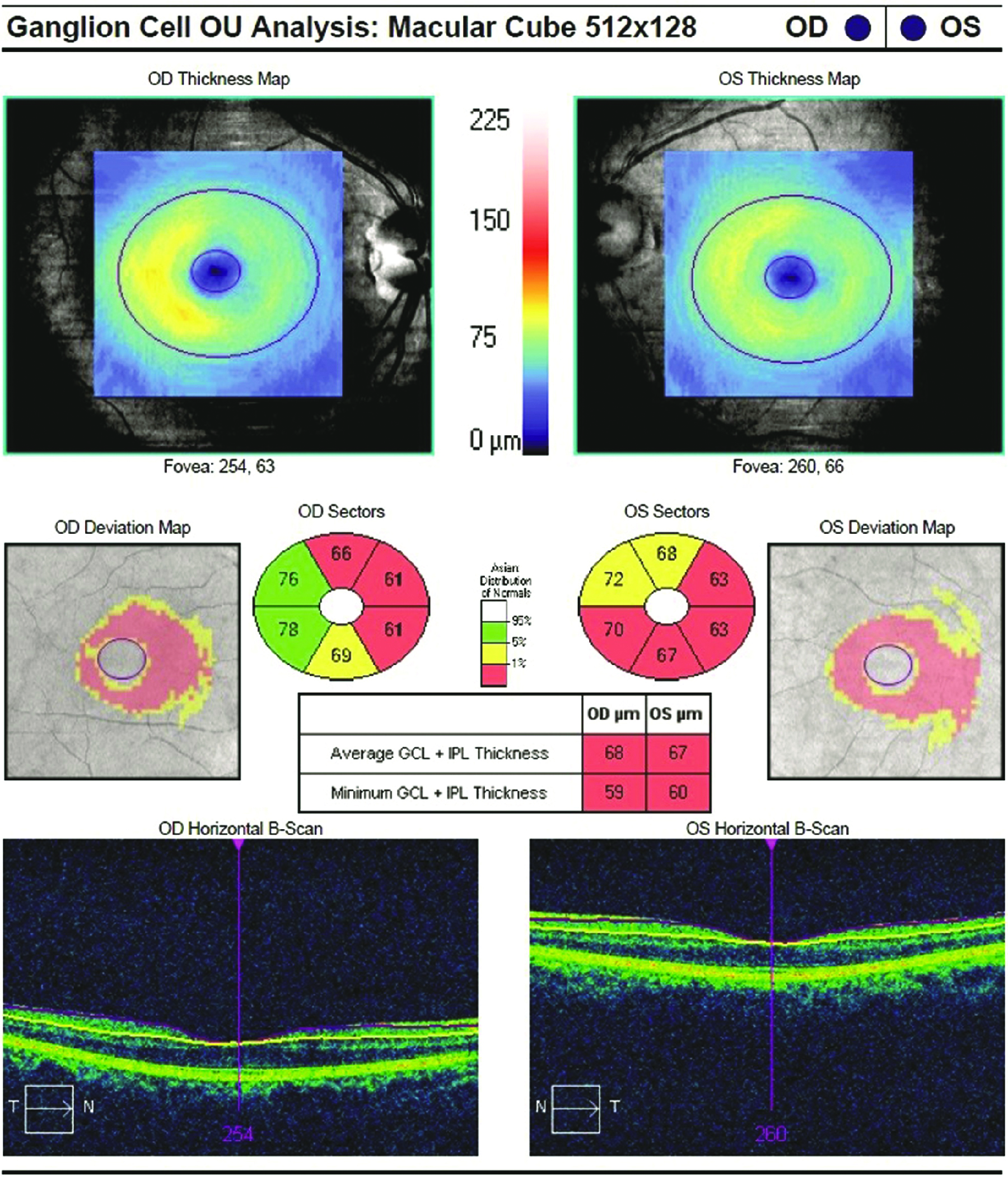
GCL and IPL thinning in an Alzheimer’s patient. Photo: Lori Mandy Pennington, OD. Click image to enlarge. |
Although much is still unknown about the development and manifestation of Alzheimer’s disease (AD), one new study points to the condition affecting more than has typically been documented. Commonly known to affect the brain structures involving learning and memory, including the hippocampus and entorhinal cortex, one study systematically reviewed articles mentioning Alzheimer’s in relation to its effects on the retina.
The study sifted through PubMed, BVS, Scopus and the Cochrane Library, using terms in conjunction to both the retina and AD to find articles that outlined the relationship of retinal and cerebral alterations. Included in the review were 23 articles that studied the retina in relation to neuroimaging. The patients of these studies had Alzheimer’s, mild cognitive impairment (MCI) and preclinical AD.
Most of the studies corroborated the relationship between the AD and the retina. Neuroimaging findings included retinal changes in relation to beta-amyloid (Aβ) deposition, a hallmark characteristic of AD. Decreased brain volume of the hippocampus, entorhinal cortex and parietal cortex also displayed a relationship to retinal structure. Specific to the visual pathway, the occipital lobe displayed higher increments of white matter hyperintensities.
OCT has also found a great deal. Reduction of thickness of the RNFL, ganglion cell layer and inner plexiform layer (IPL) have been linked to reduced brain volume in areas like the hippocampus and occipital lobe. OCT-A offered a different kind of finding, signaling a relationship to retinal vascular alterations in those with AD, MCI or preclinical AD.
One longitudinal study found dementia was associated with a thinner ganglion cell complex and IPL. Even further, a thinner baseline RNFL was additionally associated with increased risk of developing dementia.
The authors do provide their thoughts as to what mechanism causes these relationships. It’s possible that Aβ and tau deposition of the visual pathway in the occipital lobe causes retrograde neuronal degeneration of the optic nerve, as well as retinal sublayer deterioration.
This may be a breakthrough for earlier diagnosis of AD. Having ODs use OCT to potentially identify a retinal biomarker of Alzheimer’s would be a big step from the disease’s usual diagnostic pattern, which is generally identification after patients are already exhibiting challenges or some memory issues. Of course, the authors note that “more rigorous research is needed in the field, including homogenized, longitudinal and prolonged follow-up studies, as well as studies that include all stages of AD.”
Despite this early stage of discovery, they conclude that “this will enable better understanding of the retina and its implications in AD, leading to the discovery of retinal biomarkers that reflect brain alterations in AD patients in an accessible and noninvasive manner.”
Carazo-Barrios L, Cabrera-Maestre A, Alba-Linero C, et al. Retinal neurodegeneration measured with optical coherence tomography and neuroimaging in Alzheimer disease: a systematic review. J Neuro-Ophthalmol. 2022. [Epub ahead of print]. |

