 |
A 50-year-old male presented with slowly progressive blurred vision in his left eye, which he said he’d experienced over the past few years. He reported his right eye has been “bad” for at least 10 years and that he was diagnosed with a retinal condition when he was 27 years old. That primarily affected his right eye, but he believed the left was becoming significantly affected.
On exam, his best-corrected visual acuity was 20/400 OD eccentrically viewing and 20/60 OS. Confrontation visual fields were full-to-careful finger counting OU. His ocular motility testing was normal, and the pupils were equally round and reactive to light without an afferent pupillary defect. The anterior segment was unremarkable. His tensions measured 16mm Hg OU.
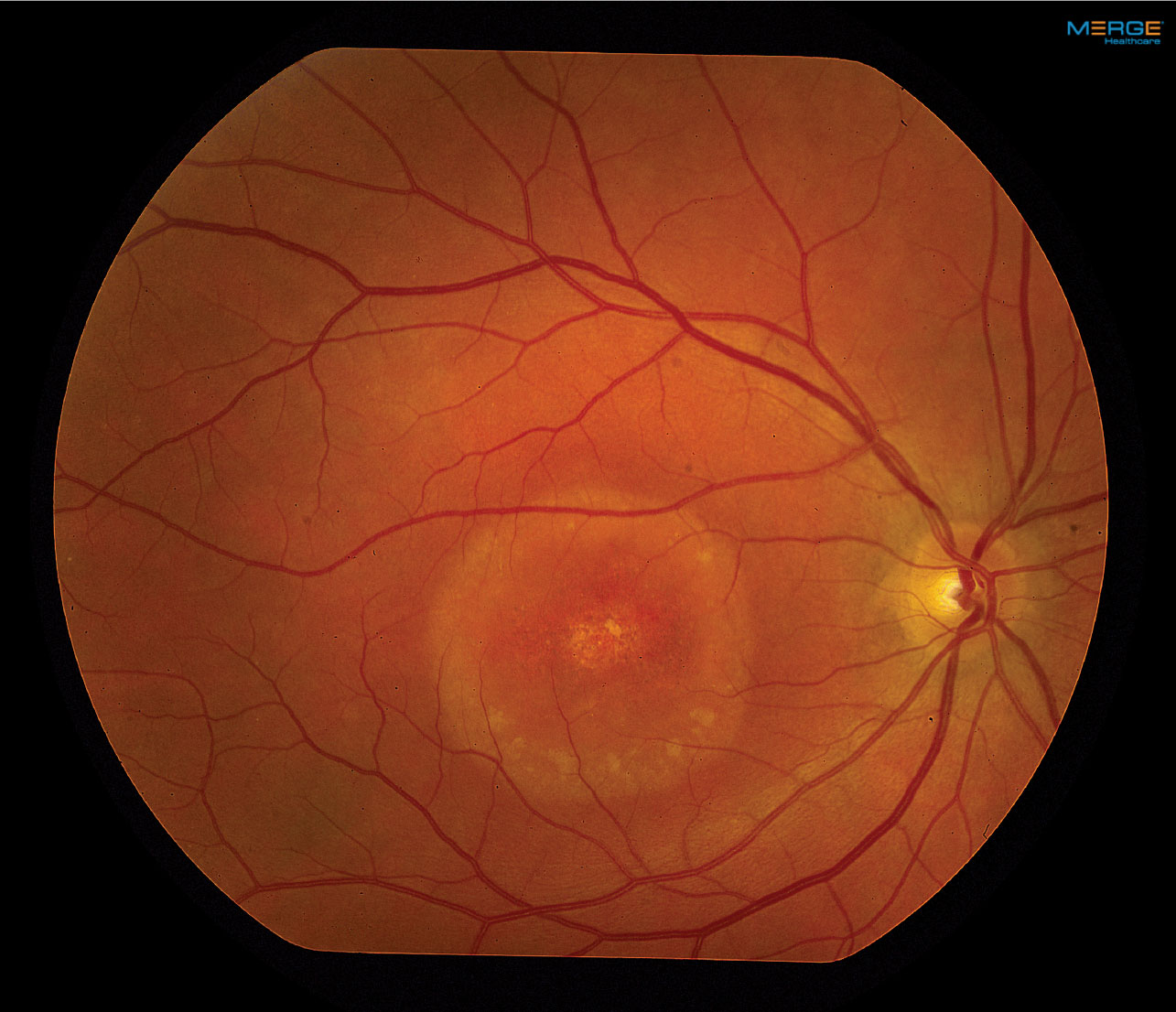
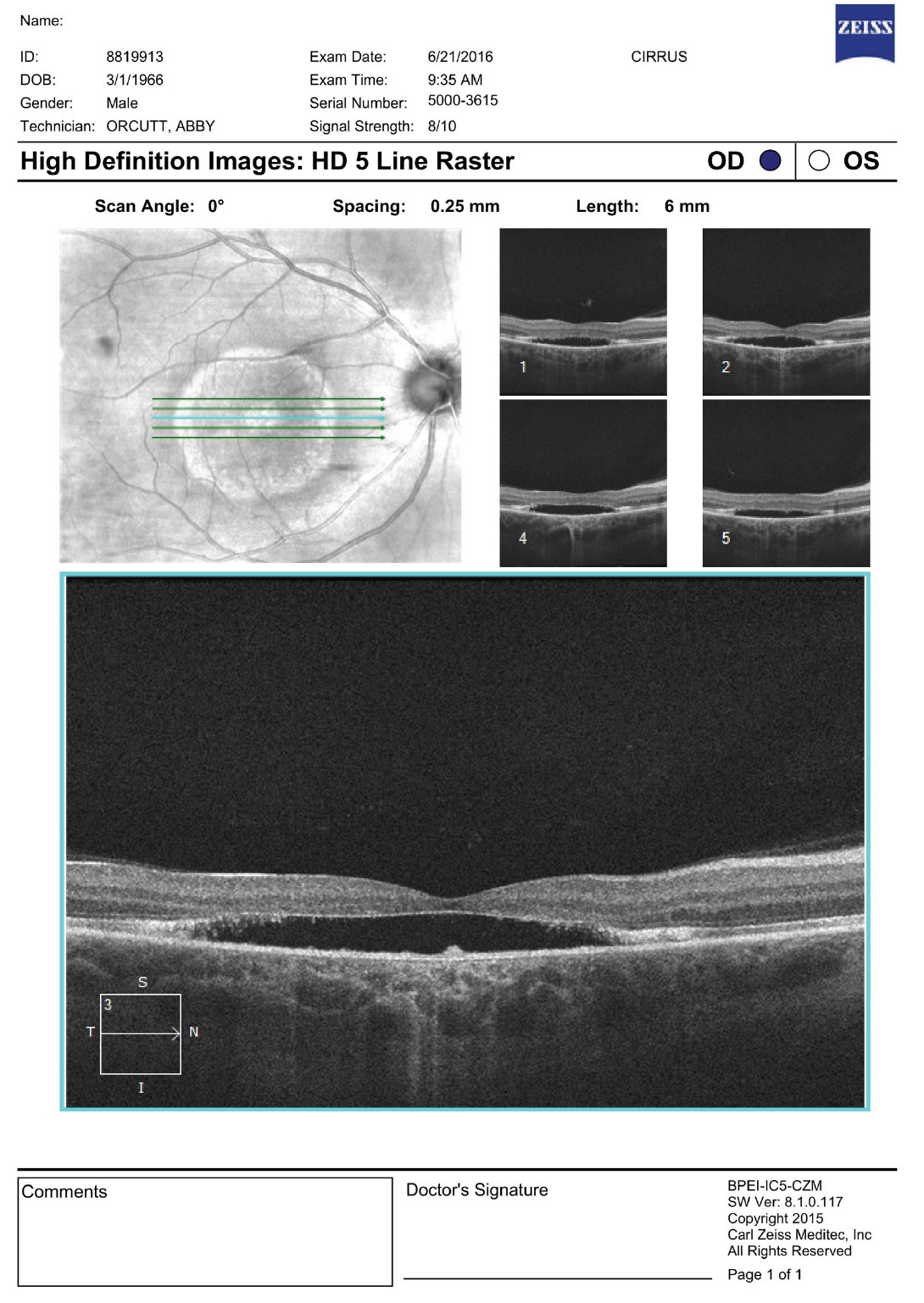
On dilated fundus exam, the vitreous was clear, optic nerves appeared heathy with small cups and good rim coloration and perfusion. Obvious retinal changes were seen in the macula of each eye (Figure 1). Optical coherence tomography (OCT) was also performed (Figure 2).
 |
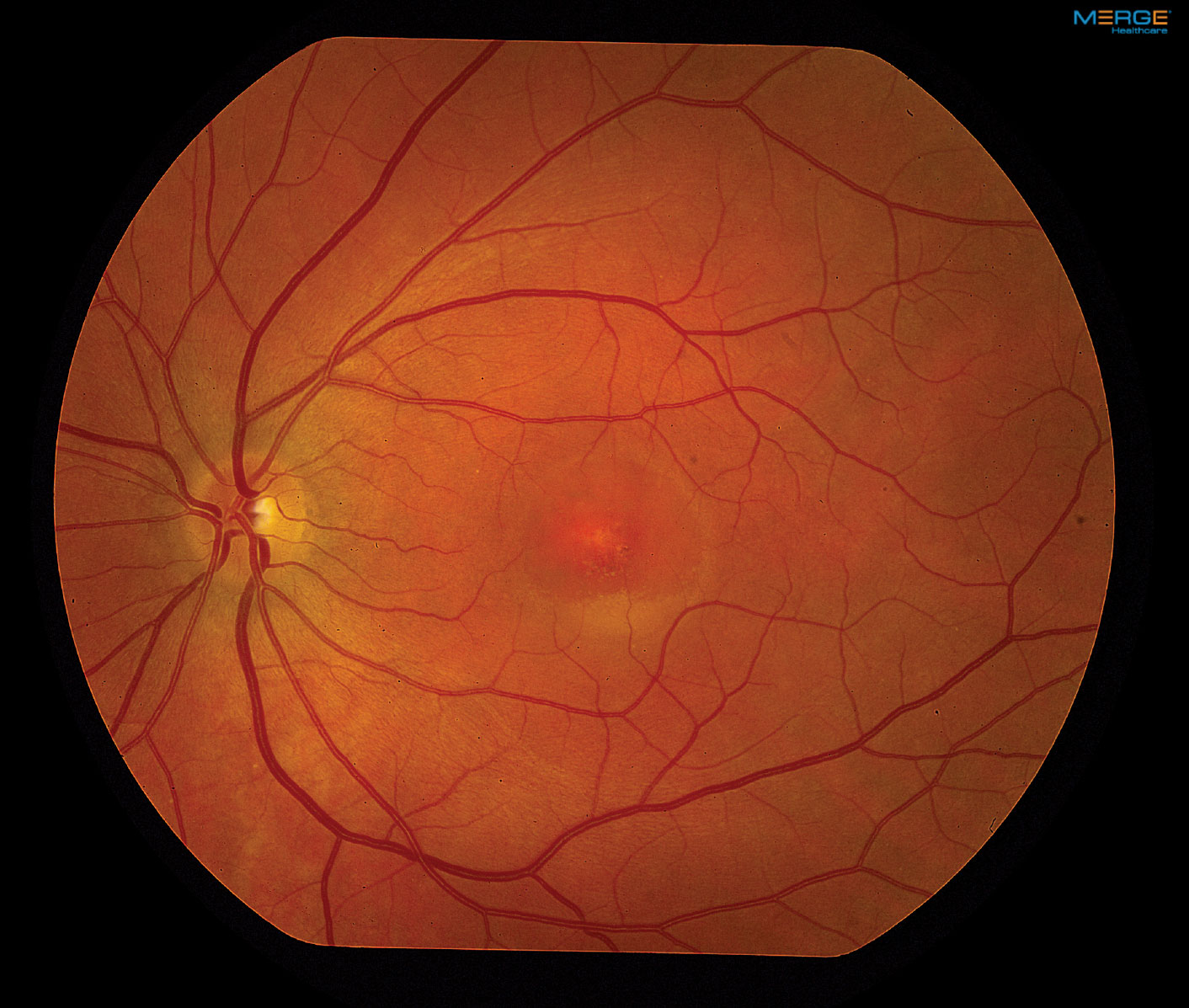 |
| Fig. 1. Our patient’s fundus photos reveal changes in both maculae. Click images to enlarge. |
Take the Retina Quiz
1. What is the most likely diagnosis?
a. Central serous chorioretinopathy.
b. Best’s vitelliform macular dystrophy.
c. Adult-vitelliform macular dystrophy.
d. Cone dystrophy.
2. What are the essential findings on the OCT?
a. Neurosensory retinal detachment and loss ellipsoid zone.
b. Retinal pigment epithelial detachment.
c. Choroidal neovascularization.
d. Macular schisis.
3. What is the likelihood that any siblings he has might have the same condition?
a. Almost no chance. It’s not hereditary.
b. About a one in 10 chance his siblings would have it.
c. 50% chance.
d. Greater than 90% chance.
4. What is the prognosis?
a. Stability with no effect on visual function.
b. Slow, steady progression and loss of central vision in his left eye.
c. Return to normal vision following treatment.
d. Complete blindness.
 |
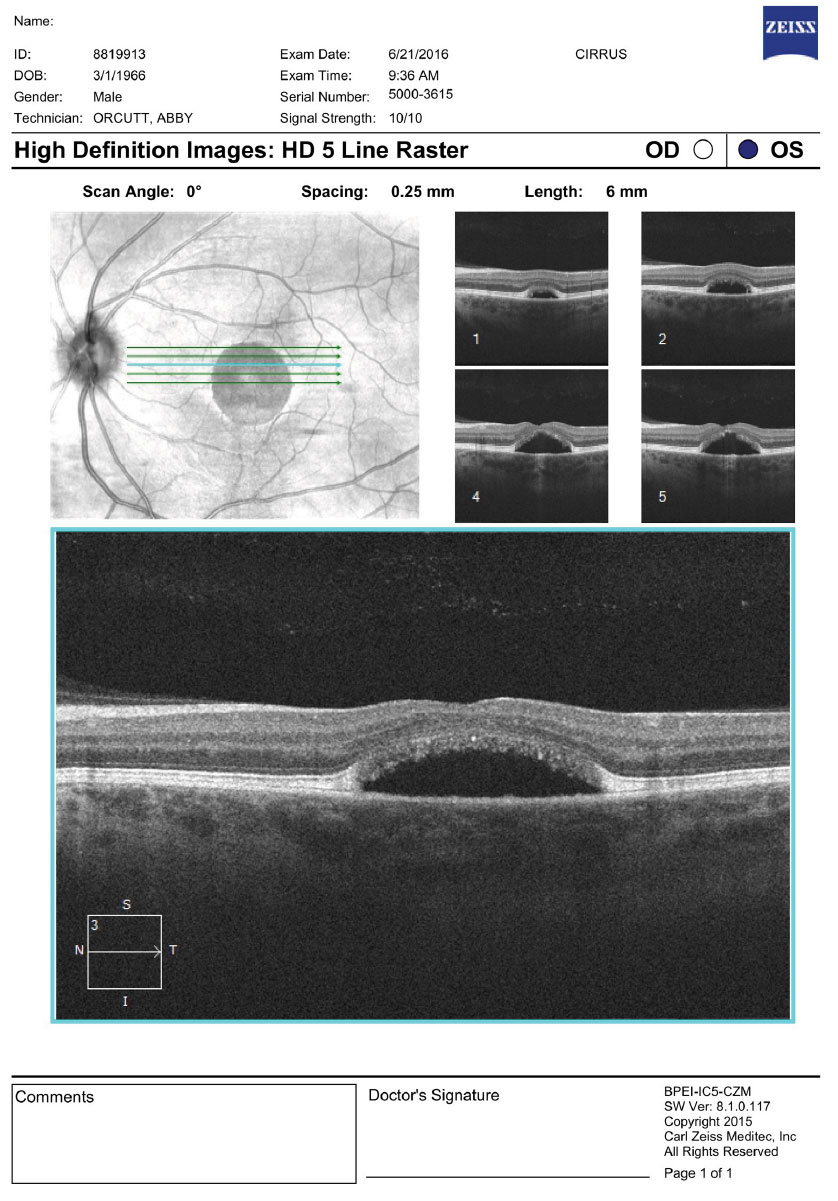 |
| Fig. 2. OCT images of the right and left macula. What are the striking features? Click images to enlarge. |
Diagnosis
The macular appearance in both eyes was quite striking, especially the right eye where central retinal pigment epithelial (RPE) depigmentation and atrophy were clearly visible. Surrounding the macula we noted a circumferential ring that had a bull’s eye pattern. The left eye also had central RPE atrophic changes, but clearly not to the extent of the right eye. The patient appeared to have a neurosensory detachment that was visible on both the clinical exam and OCT. Inferior to the macula the almost hypopyon-like appearance helped make the diagnosis.
The family history also provided a clue to the diagnosis—his sister reportedly has the same condition, and his mother is a carrier. Our patient was diagnosed when he was 28 years old. So what does this all add up to? Our patient has Best’s vitelliform macular dystrophy (BVMD), an autosomal dominant hereditary retinal dystrophy that affects the retinal pigment epithelium. Vision is usually not affected until childhood or early adulthood and generally has a good prognosis for maintaining good central vision in at least one eye.
The classic description for BVMD is the bilateral yellow egg-yolk appearance of the macula. At one point our patient had that appearance, but as the disease evolves the appearance can change. BVMD is classified by five stages. (see BVMD staging).
Our patient’s right eye has progressed from Stage IV to Stage V because of the atrophic changes in the fovea, but you can still see remnants of the tale tell sign of Stage IV BVMD, which is more like a “scrambled egg” appearance around the macular than Stage II, which resembles an “egg yolk.” His left eye is in stage III, as the pseudophyopyon is clearly apparent with layering of the lipofuscin material. It is likely that his left eye will experience a slow progression. About 20% of patients will develop choroidal neovascularization (CNV) following the atrophic stage which can further have an effect on central acuity.1,2
Research links BVMD to a genetic mutation on the BEST1 gene, which is located on chromosome 11 (11q12.3).3 It encodes for a transmembrane protein bestophin 1 which is believed to affect the conductance of chloride which negatively affects the transport of fluid across the RPE. This in turn results in the accumulation of debris between the RPE/photoreceptor complex and Bruch’s membrane.
The diagnosis can usually be made based on clinical presentation. When in doubt, an electrooculogram is diagnostic and will be positive even in the previtelliform stage when the retina appears unaffected and the vision is normal.
Best’s Vitelliform Macular Dystrophy Staging
|
Monitoring
Mutimodal imaging, including fundus photography, OCT and fundus autofluoresence (FAF), may be helpful in characterizing BVMD. OCT can show classic structural changes within the retina. Even in the previtelliform stage, thickening and hyper-reflectivity of the RPE/ellipsoid zone (IS/OS junction) can be seen. In the vitelliform stage, a homogenous hyper-reflective band will be present, which represents the vitelliform material that is deposited in the outer retinal layers. This is thought to be the accumulation of the photoreceptor outer segments containing lipofuscin.4 As the disease progresses and atrophy develops, loss of the ellipsoid junction will occur in the macula; outside the macula, the hyper-reflected band can still be present as we see in our patient. The hyper-reflective area in the right eye represents the white band that we described as a bull’s eye in appearance.
It is unknown if the serous detachment seen in both eyes truly represents a detachment of the sensory retina from the RPE, or more likely a separation of the RPE from its underlying attachment to Bruch’s membrane leaving a central subfoveal hypolucent space.
No genetic treatments available yet for BVMD. Our patient was able to maintain good central acuity in the left eye for many years. Five years earlier, at 45 years old, his acuity was still 20/25 and he was able to drive and was functioning without difficulty. Over the following five years, he has experienced a slow decline in acuity that affected his quality of life. We referred him to low vision services and issued him stronger reading glasses over his contact lenses.
1. Ryan S. Retina, 4th ed. In: Schachat AP, ed. Volume II; Medical Retinal. Philadelphia. Elsevier;2006. 2. Gass J. Stereoscopic atlas of macular diseases. Diagnosis and Treatment. 4th ed, Mosby, St. Louis;1997:304-11. 3. Stone E, Nichols B, Streb L, et al. Genetic linkage of vitelliform macular degeneration (Best’s disease) to chromosome 11q13. Nat Genet. 1992;1(4):246-50. 4. Battaglia Parodi M, Iacono P, Romano F, Bandello F. Spectral domain optical coherence tomography features in different stages of Best vitelliform macular dystrophy. Retina. 2018;38(5):1041-6. |
Retina Quiz Answers:
1) b; 2) a; 3) c; 4) b

