 |
|
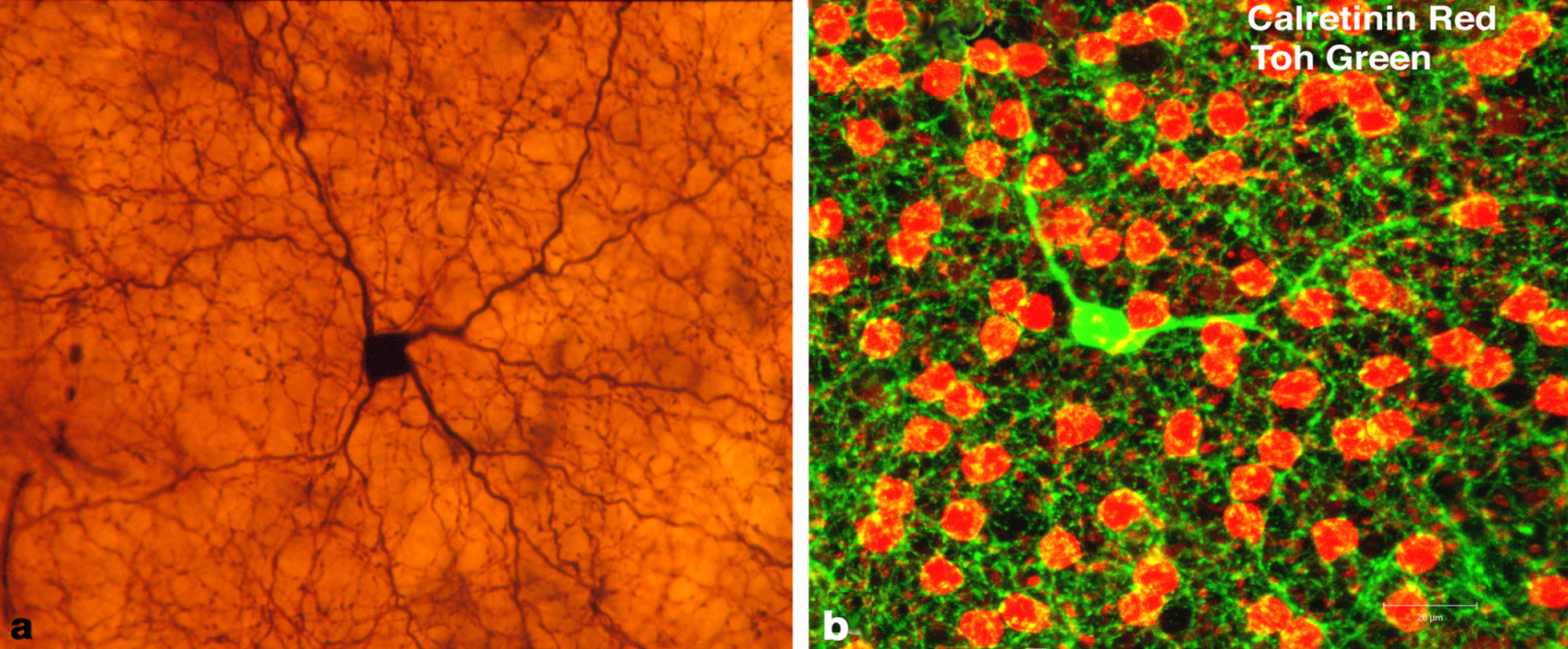
Dopaminergic amacrine cells in animal retina histology samples. Present in both the inner and outer plexiform layers, such cells may be a target for myopia control therapies in the future. Photo: Nicolas Cuenca, webvision.med.utah.edu. Click image to enlarge. |
The dopamine precursor levodopa has been used clinically for over 50 years as the primary treatment for neurological disorders characterized by diminished dopamine release, most notably Parkinson's disease. Levodopa is commonly coadministered with carbidopa to prevent its premature conversion to dopamine before reaching the target tissue, enhancing treatment outcomes. Although its anti-myopia effects have yet to be assessed in humans, a topical formulation of levodopa/carbidopa shows significant potential as a myopia treatment through work in animal models, eliciting a high degree of protection against experimental myopia.
Findings of a first-in-human Phase I trial have shown that levodopa/carbidopa topical ophthalmic solution appears to be safe and tolerable to the eye and may be worth building upon in Phase II trials for the treatment of myopia.
“One of the earliest and most consistent biochemical changes implicated in animal models of myopia is a reduction in retinal dopamine release,” the researchers explained in their paper on the work for the journal Clinical and Translational Science.
The trial group reformulated levodopa/carbidopa into a topical ophthalmic solution for direct application to the eye. The study, based in Australia, recruited 29 men between the ages of 18 and 30 (mean age: 24.9). The participants were randomly assigned to receive either a low (1.4:0.34 µmmoles/day, n=14) or standard dose (2.7:0.68 µmoles/day, n=15) of levodopa/carbidopa eye drops in one eye and placebo in the fellow eye once daily for four weeks.
Over the four-week trial, and after four months of follow-up, the levodopa/carbidopa treatment had no significant effect on measures of ocular tolerability, anterior surface integrity, visual function, ocular health, refraction or ocular biometry. No adverse events occurred over the course of the trial, and participants did not report any systemic symptoms associated with levodopa/carbidopa during treatment.
The researchers now believe that the treatment efficacy of levodopa/carbidopa can now be assessed in myopic patients. “If effective, this would provide a novel therapeutic intervention for myopia that may show significant benefits over, or complement, current treatment options,” they wrote.
The team suggested that the applicability of topical levodopa may extend beyond myopia treatment, with other studies finding systemic levodopa treatment to be beneficial in enhancing visual function in amblyopia and diabetic retinopathy and in inhibiting the release of VEGF in the retinal pigment epithelium and limiting anti-VEGF injections in AMD.
“These results indicate that topical levodopa/carbidopa is safe and tolerable to the eye,” the researchers concluded in their paper. “If found to be effective, this will provide a powerful new method for inhibiting the onset and development of the leading cause of low vision worldwide.”
Thomson K, Karouta C, Sabeti F, et al. The safety and tolerability of levodopa eye drops for the treatment of ocular disorders: a randomized first-in-human study. Clin Transl Sci. October 11, 2022. [Epub ahead of print]. |

