 |
There has been a paradigm shift in the management of glaucoma over the last decade. With the introduction of the iStent (Glaukos) back in 2012, along with the renewed interest in first-line selective laser trabeculoplasty (SLT), and now drug delivery in the form of Durysta (intracameral bimatoprost, Allergan), clinicians are appreciating the importance of earlier intervention and the impact of compliance on long-term glaucoma control. In our practice, compliance with topical glaucoma medications has been the most significant barrier to preventing glaucoma progression.
 |
|
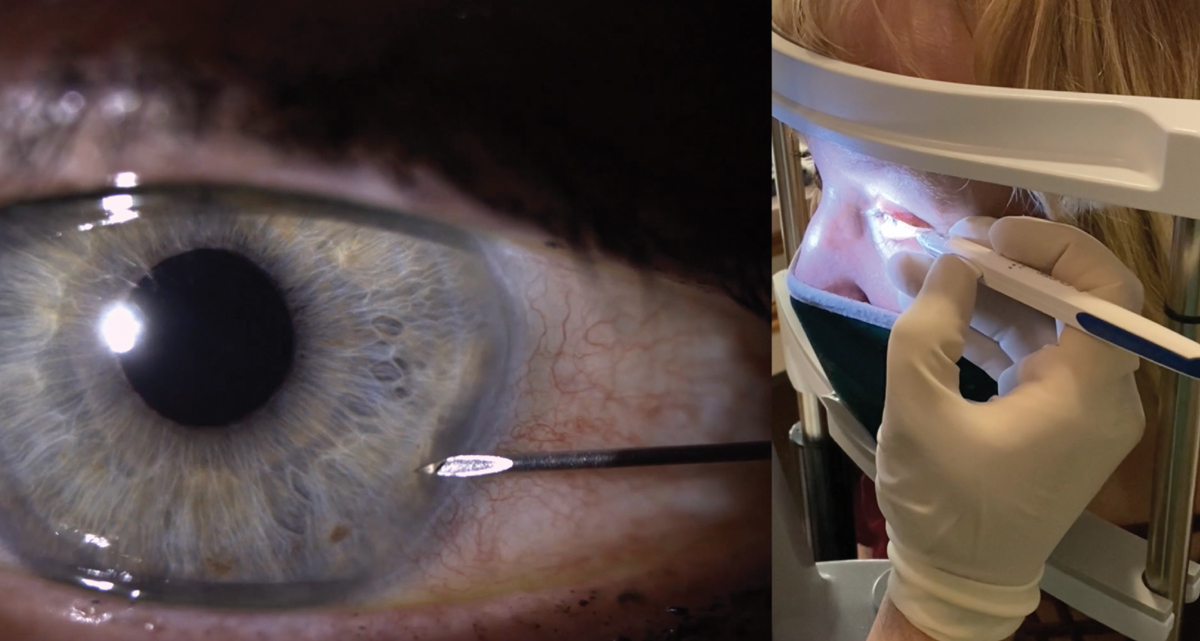
Fig. 1. Proper approach entry of the needle through the cornea. Notice the needle enters through the cornea at approximately the four to five o’clock position in this left eye. Proper hand placement showing stabilization of the hand and fingers on the patient’s face (at right). Click image to enlarge. |
The Burden
A 2015 study of over 1,200 newly diagnosed glaucoma patients who were started on topical drug monotherapy found only 20% had good treatment adherence at one year.1 Studies have found up to 60% of glaucoma patients had a concomitant dry eye, which has also been related to the number of topical glaucoma medications.2-4 Due to the symptoms of dry eye and ocular surface disease (OSD) such as tearing, burning, pain and fluctuating vision, patients often blame their glaucoma drops and then reduce or even stop using them. Addressing compliance is crucial since poor compliance can lead to fluctuating intraocular pressure (IOP).
With the importance of reducing the topical drop burden for patients known, where do anterior chamber implants, in the form of sustained-release glaucoma medications, fit into the glaucoma treatment paradigm? Since June 2020, eyecare providers have had access to the Durysta intracameral implant, which has allowed us to decrease the drop burden in a variety of patients in multiple situations. Durysta is a 1mm preservative-free implant infused with 10µg of bimatoprost, which actively releases medication 24 hours per day for approximately four months. It is a preloaded device with an ergonomic injector system.
This procedure is within the scope of practice of optometrists in a few states. Regardless of whether you are in a state where ODs can perform the actual injection or you are comanaging Durysta with your local ophthalmologist, it is imperative that optometrists be familiar with the ins and outs of this procedure that can ease the drop burden for your patients.
 |
|
Fig. 2. A secondary device, a cotton-tipped applicator, is being used to apply a little countertraction opposite the needle as the procedure is about to be performed. Click image to enlarge. |
The Data
The ARTEMIS 1 and ARTEMIS 2 Phase III clinical trials were two multicenter, randomized, parallel-group, controlled studies comparing the 10µg bimatoprost implant to twice-daily timolol 0.5% topical drops.
Parallel groups of patients diagnosed with OAG or ocular hypertension (OHT; with a baseline IOP of 22mm Hg to 32mm Hg) were followed for a period of 20 months, including an eight-month extended follow-up. Durysta lowered mean IOP by 30% to 33% from baseline over the 12-week primary efficacy period. This works out to be about 5mm Hg to 8mm Hg of reduction from the mean baseline IOP of 24.5mm Hg.5 The bimatoprost implant met predefined criteria for non-inferiority compared with timolol.
Patient Selection and Pre-op Considerations
The indication for Durysta is very broad. It is FDA-cleared as a single implant for patients with OAG or OHT. This includes primary open-angle glaucoma (POAG) and even secondary open-angle glaucomas such as pigmentary, pseudoexfoliation, angle recession or steroid-induced glaucoma. It can be considered in these selected patients regardless of stage of disease, be it mild, moderate or severe.
Gonioscopy is critical in the evaluation for consideration of Durysta. An open angle needs to be confirmed to ensure there is enough room in the space for the bimatoprost implant to sit in the inferior angle without touching or rubbing up on the cornea.
Talking to the patient regarding their struggles with eyedrops—whether it be side effects, cost, convenience, forgetting to administer or physical limitations to instillation—is important to gauge their motivation for alternative options such as Durysta.
 |
|
Fig. 3. The needle is now in the anterior chamber at the proper position for insertion of the pellet, about two bevel lengths into the anterior chamber. Notice how the needle is properly placed in front of the iris. Click image to enlarge. |
It is critical that patients understand the nature of the procedure, how it is done, and the benefits and limitations (such as the effectiveness will not last forever, and currently it is FDA approved for a single implant only). So, how do we discuss this anterior chamber implant with our patients? Once you have identified a compliance-related issue, use the idea of “compliance” as the rationale to discuss the implant. You are offering a solution to their problem.
A consent form that details the nature of the procedure, single administration of the procedure, temporary nature of the effect of the IOP lowering (typically four months to two years), risks and potential complications and alternative treatments, should be thoroughly reviewed with the patient and the consent form signed.
Preoperative drops on the day of the procedure include:
• One to two drops of a topical ophthalmic anesthetic (proparacaine) in both eyes to minimize the blink reflex.
• One to two drops of a topical ophthalmic antibiotic in the procedure eye.
• One to two drops of 5% ophthalmic povidone-iodine (Betadine) to ensure proper asepsis.
The Procedure
Durysta implantation can be performed in the office at the slit lamp or in a minor procedure room with the patient in the supine position. It can also be done at the ambulatory surgical center or hospital operating suite.
Regardless of where the procedure is performed, it is important to follow three important principles:
1. Use good magnification, whether loops, a slit lamp or a microscope.
2. Provide good patient head stabilization, i.e., having a technician hold the patient’s head still if performed at the slit lamp.
3. Follow good aseptic sterile technique, using 5% povidone iodine prep, sterile gloves and the option of periprocedural topical antibiotics.
 |
|
Fig. 4. The actuator button is being depressed, and the implant is starting to be released from the device. Click image to enlarge. |
Below are basic steps to guide the practitioner on the slit lamp implantation technique:
• Anesthetize the nonprocedure eye as well. This will prevent the patient from blinking either eye.
• Have the patient and the slit lamp in proper position before removing the device from the packaging.
• Once you have removed the device from the packaging, remove the safety tabs of the device.
• Rest your hand on the cheek of the patient to stabilize the device. Using an elbow rest is also encouraged (Figure 1).
• Have the patient focus on one part of the slit lamp or its fixation target. This will provide natural countertraction. Additionally, you may want to use a second instrument (cotton tipped applicator or 0.12mm forceps) to stabilize the eye. You can apply a little counter-traction using a soft cotton tip swab positioned 180° away from where you enter with the device needle. The second instrument can help to generate enough counterforce for a straightforward entry into the anterior chamber (AC) (Figure 2).
• The insertion is performed by entering the AC via the loader’s 28-gauge needle through the clear cornea, engaging just anterior to the limbal vessels.
• The goal is to enter the AC parallel to the iris and maintain the needle over the iris for the entire procedure.
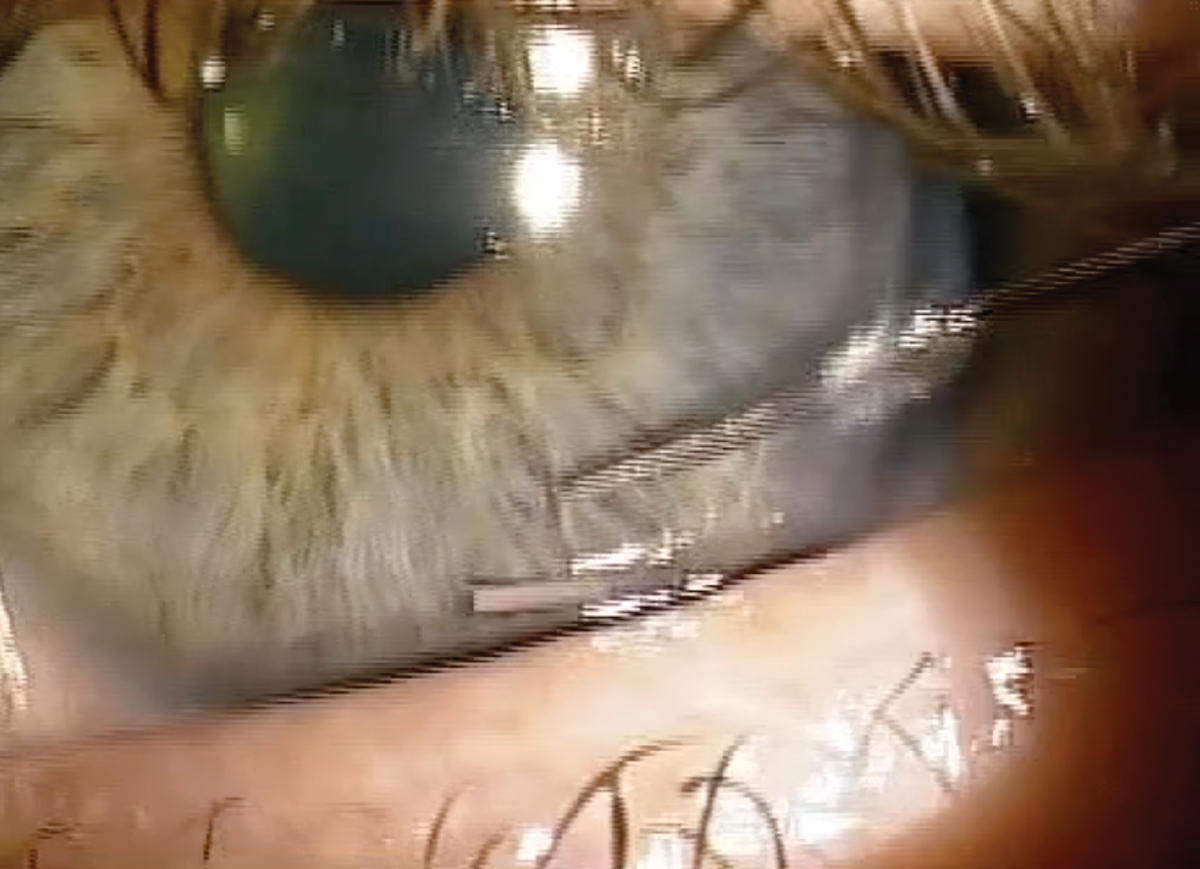
• One should enter at the clock hour that is most convenient for your hand position and the anatomy of the orbit and eye. I tend to enter around seven or eight o’clock for the right eye and four or five o’clock for the left eye (Figure 3).
• It is recommended when inserting Durysta in the right eye to use your left hand to hold the device, which allows you to enter temporally near the seven or eight o’clock position, whereas for the left eye you will want to use your right hand to enter near the four to five o’clock position. Entering at these clock hour positions will allow you to enter directly perpendicular to the cornea and maximize the area over the iris.
 |
|
Fig. 5. The actuator button has been fully depressed, and the implant has been released and immediately falls towards the inferior angle. Click image to enlarge. |
• After entering and ensuring you are approximately two needle bevel lengths into the AC, press the posterior aspect of the actuator button, which is located on the device, to release the implant. Once the implant is released (Figures 4 and 5), come straight back out of the eye. It is important to remove the needle straight back out of the track to avoid tangential forces on the wound, which could cause aqueous to escape and the implant to migrate back to the wound. Also, make sure your thumb is already on the actuator button so you can press it without having to take your eyes away from the slit lamp oculars. One pearl: press the oval button with conviction, for if you are too slow, the implant may get stuck to the needle.
• Check the wound using a surgical spear sponge or cotton-tipped swab to check for a wound leak. Place a drop of antibiotic in the eye after the procedure in-office. An antibiotic prescription for the patient to take home is not necessary.
• With gravity, the implant sinks to the inferior angle, where it resides (Figure 6) and slowly releases the bimatoprost over the course of the next four months.
• The majority of clinicians implant Durysta in one eye, then have the patient come back one to two weeks later for implantation of Durysta in the fellow eye.
 |
|
Fig. 6. Gonio view of the inferior angle showing perfect position of the Durysta pellet a few minutes after the procedure was completed. Click image to enlarge. |
Key Post-op Considerations
• Topical ophthalmic antibiotic eyedrop instilled in office immediately after the procedure.
• Patient education to teach them to:
–Keep head elevated for the first day.
– Not rub the eyes for the first 48 to 72 hours.
• Return to clinic in four to six weeks for an IOP check and gonioscopy to check positioning of the implant.
Proper management of glaucoma with IOP readings within our target ranges is key to providing the best possible long-term outcomes for our patients. Noncompliance with eye drops is one of most significant barriers patients and eye care practitioners face in glaucoma.
Administration of intracameral bimatoprost into the anterior chamber has provided relief and eased the burden for many patients. The idea of interventional glaucoma, where noncompliance is less of an issue and IOP is more controlled with procedures such as SLT, MIGS or Durysta implants, is only just gaining steam and will likely continue to evolve as a bigger part of our glaucoma treatment armamentarium.
All eyecare practitioners, whether you plan to do the procedure in the future or refer to another provider, are encouraged to consider the benefits of sustained drug delivery for your patient. Everyone likes a holiday, and glaucoma patients have been thrilled to have an eyedrop vacation!
1. Newman-Casey PA, Blachley T, Lee PP, et al. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122(10):2010-21. 2. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618-21. 3. Erb C, Gast U, Schremmer D, et al. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593-1601. 4. Leung EW, Medieros FA, Weinreb RN, et al. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma.2008(5);17:350-5. 5. Safety and efficacy of bimatoprost sustained-release in patients with open-angle glaucoma or ocular hypertension. clinicaltrials.gov/ct2/show/NCT02250651. Accessed May 25, 2023. |

