 |
A 52-year-old African American female reported for an annual eye exam. Her chief complaint was difficulty with reading. She indicated she had an ocular history of medullated nerve fibers in the right eye diagnosed by another practitioner many years ago. She also indicated she had a family history of glaucoma (grandmother). Her systemic history was significant for hypertension, for which she was properly medicated. She denied allergies of any kind.
Clinical Findings
Her best-corrected entering visual acuities were 20/20 OU at distance and near. Her external examination was normal with no evidence of color deficiency, brightness loss, field abnormality or afferent pupillary defect. Refraction was negligible, with improvement at near with a small increase in add power. Biomicroscopy uncovered normal and healthy anterior segment tissues with Goldmann applanation tonometry measuring 19mm Hg OU.
Her dilated funduscopic exam was within normal limits; the photograph below, from old records, demonstrates the “medullated nerve fibers” seen by the previous practitioner. Her cup-to-disc ratios were slightly asymmetric, measuring 0.4/0.4 OD and 0.4/0.55 OS.
For More Information
Additional testing included requesting the previous records and obtaining visual fields, OCT and photos to establish a baseline in case she was considered to be a borderline glaucoma suspect based upon the asymmetric C/D ratio and family history. Measurement of central corneal thickness (pachymetry) to understand the relative conversion risk to treatable glaucoma and gonioscopy to understand angle status is also recommended.
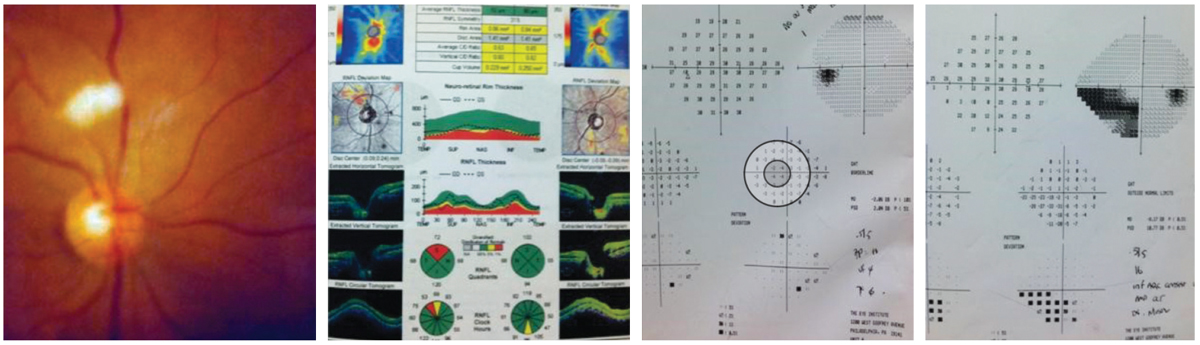 |
|
Examination and diagnostic findings in our patient. How to these correspond to her history and chief complaint? Click image to enlarge. |
Diagnosis and Pathophysiology
This is a case of inferior right quadrantanopic visual field loss secondary to nerve fiber layer loss as a result of an old nerve fiber layer (NFL) infarction (cotton wool spot) precipitated from systemic hypertension.
Hypertension (HTN) affects over 1.2 billion individuals worldwide.1 It is a multifactorial disease involving multiple environmental and genetic factors, with the cause of the disease identified in only about 10% of the cases (secondary hypertension).1 In 90% of the cases, no etiology is found (primary or essential hypertension).1
Blood pressure is linearly associated with cardiovascular morbidity and mortality and represents a major modifiable risk factor regarding the burden of stroke, cardiovascular disease and mortality worldwide.1
With respect to the eyes, it is a risk factor for vision-threatening eye conditions such as branch retinal artery occlusion (BRAO), central retinal artery occlusion (CRAO), branch retinal vein occlusion (BRVO), central retinal vein occlusion (CRVO), retinal artery macroaneurysms and non-arteritic anterior ischemic optic neuropathy (NAION).2 Hypertension has the potential to increase the development and progression of diabetic retinopathy, glaucoma and age-related macular degeneration.1-7
Early retinal findings include generalized retinal arteriole narrowing secondary to vasospasm and increased vascular tone.2 Chronic hypertension (whether poorly controlled or unmanaged) leads to structural changes in the vessel walls. These include intimal thickening and hyaline degeneration.2 This produces both focal and diffuse vessel wall opacifications, which can be seen as a distinct, visible, linear reflex upon fundus examination. These changes are known as copper or silver wiring.2,4,6
As retinal arteries often pass over retinal veins at places where they intersect, thickened arterioles compress the venules where they cross, producing what is known as arteriovenous (AV) nicking.2
All of these changes impair retinal circulation, creating areas of ischemia in the retinal nerve fiber layer (the location where the retinal arterioles are located). When axons of ganglion cells succumb, cotton-wool spots (CWS) form.2-4 These are accumulations of axoplasmic debris within adjacent bundles of unmyelinated ganglion cell axons. Their formation is widely held to reflect focal ischemia from terminal arteriolar occlusion where restricted axoplasmic flow and neural damage produces fiber swelling and the thickened white appearance of tissue that is normally transparent.2-6 CWS have also been purported to be nothing more than sentinels of retinal nerve fiber layer pathology (what is left after the damage).5
Finally, when the blood-retina barrier is damaged, blood will be released into the nerve fiber layer (“flame-shaped” retinal hemorrhages) along with the exudation of lipids (often seen near the macula and known as “circinate” maculopathy).2 Severe hypertension can lead to increased intracranial pressure, causing optic nerve ischemia and optic disc swelling.2
Hypertensive choroidopathy is usually seen in combination with hypertensive retinopathy and is termed hypertensive chorioretinopathy.2 This condition is characterized by necrosis of choroidal arterioles with resultant non-perfusion of the overlying choriocapillaris. This produces ischemic damage to the retinal pigment epithelium, which can be observed clinically; the phenomenon is known as Elschnig spots.2 Over time, these lesions become irregularly pigmented. Larger areas of choroidal ischemia along larger choroidal lobules appear as linear hyperpigmented streaks, which trace the course of the underlying choroidal arteries they affect. These are called Siegrist streaks.2
Ruling out Glaucoma
Without the initial photograph (which was misdiagnosed as medullated nerve fibers), there was no way to know this was not in fact either optic neuropathy or some manifestation of glaucomatous loss. The patient was treated immediately with a topical prostaglandin medication with the goal of lowering the IOP by 30% and allowing the nerve to perfuse against less resistance. A prompt referral was made to a glaucoma service to rule out normal tension glaucoma and to assess the need for additional systemic testing; neuroimaging was ordered to rule out non-glaucomatous sources of optic neuropathy.
The glaucoma specialist agreed with our interpretations and plan and initiated the appropriate workup, which proved negative. After the true cause of the deficit was determined, following a detailed discussion, the patient asked to remain on therapy for “peace of mind.” Neither we nor the glaucoma specialist had an issue with this strategy. The patient remains stable to this day, perfusing against less resistance with no further losses.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. Brown GC, Brown MM, Hiller T, Fischer D, Benson WE, Magargal LE. Cotton-wool spots. Retina. 1985 Fall-Winter;5(4):206-14. 2. Tsukikawa M, Stacey AW. A Review of Hypertensive Retinopathy and Chorioretinopathy. Clin Optom (Auckl). 2020;12(5):67-73. 3. Alencar LM, Medeiros FA, Weinreb R. Progressive localized retinal nerve fiber layer loss following a retinal cotton wool spot. Semin Ophthalmol. 2007;22(2):103-4. 4. McLeod D. Why cotton wool spots should not be regarded as retinal nerve fibre layer infarcts. Br J Ophthalmol. 2005;89(2):229-37. 5. Rossier BC, Bochud M, Devuyst O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology (Bethesda). 2017;32(2):112-125. 6. Grosso A, Veglio F, Porta M, Grignolo FM, Wong TY. Hypertensive retinopathy revisited: some answers, more questions. Br J Ophthalmol. 2005;89(12):1646-54. 7. Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD006127. |

