 |
A 65-year-old Caucasian male presents as a new patient to establish care in May 2018 with complaints of gradual, yet painless, blurry vision in his left eye over several months. His last reported visit to an eye doctor was approximately five years earlier. Current medications included Tenormin (atenolol, AstraZeneca), omeprazole, Zocor (simvastatin, Merck) and multivitamins. He reported no known allergies to medications.
Diagnostic Data
At this initial visit, entering visual acuities were 20/30 OD and 20/60 OS, best corrected to 20/20 OD and 20/50+ OS. There was no frank afferent pupillary defect (APD) noted, and his extraocular muscles were full in all positions of gaze. Confrontation fields were full in both eyes.
A slit lamp examination of his anterior segments was unremarkable, with open anterior chamber angles and clear corneas. Applanation tensions were 14mm Hg OD and 13mm Hg OS at 10:45am. Pachymetry readings were 511µm OD and 513µm OS.
Through dilated pupils his crystalline lenses were clear, with perhaps some incipient nuclear changes OU, but not significant enough to affect vision. The anterior vitreous OU was clear.
Findings
The posterior segment was characterized by clear maculae, and retina vasculature characterized by moderate arteriolarsclerosis, consistent with his medical history of hypercholesterolemia. The cup-to-disc ratios were 0.35 x 0.45 OD and 0.45 x 0.65 OS, with a somewhat thinned neuroretinal rim from 1 o’clock to 5 o’clock. I noted some mild pallor of the temporal aspect of the left optic nerve, and margins in both eyes were distinct. His peripheral retinal evaluation was normal.
Following the posterior pole examination, and the optic nerves in particular, I reviewed with him his history and, although it was not the most detailed, there appeared to be no distinct, acute onset of decreased vision consistent with a non-glaucomatous optic neuropathy, such as non-arteritic ischemic optic neuropathy. He attributed the change in his vision to both age and a lapse in routine eye care for several years. He denied any phosphodiesterase type 5 (PDE-5) inhibitor use. Essentially, a review of this aspect of the history was not forthcoming in details suggestive of acute optic neuropathy.
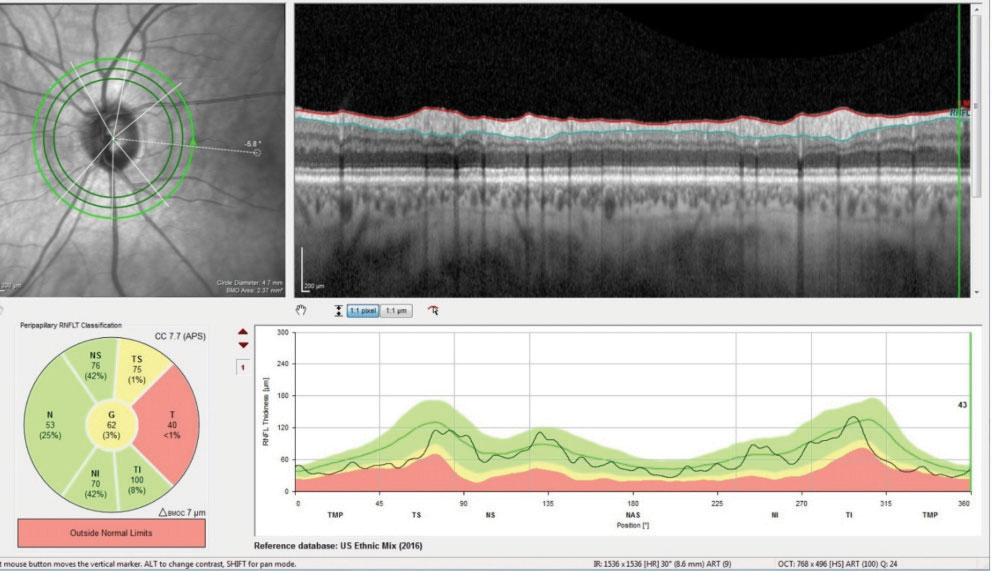 |
| Fig. 1. These readouts demonstrate the global reduction in the temporal neuroretinal rim BMO complex consistent with a previous episode of unspecified non-glaucomatous optic neuropathy. |
Diagnosis
Accordingly, the patient was initially diagnosed as a normal tension glaucoma suspect, more so in the left eye than the right, with the possibility of an old ischemic optic neuropathy in the left. He was prescribed glasses to get him to best-corrected visual acuity levels, and he was asked to return in a few weeks for optic nerve imaging and threshold visual field studies.
At that follow-up visit, close examination of his pupillary reactions yielded an equivocal APD on the left side. Neutral density filters were missing in the office, so no sensitivity pupillary testing could be done.
Applanation tensions were 12mm Hg OD and 13mm Hg OS. Threshold flicker-defined form (FDF) visual field testing of his right eye demonstrated an essentially normal field. The left eye was consistent with a relatively small central defect, along with pericentral areas consistent with the thin inferotemporal neuroretinal rim, suggestive of early glaucomatous loss, but this area was contiguous with the central defect.
Heidelberg retina tomography (HRT 3) and optical coherence tomography (OCT) imaging of the optic nerves were obtained. The HRT 3 imaging confirmed the initial estimate of the neuroretinal rim contours with the left eye, showing thinning of the temporal rim. The OCT evaluation—using the glaucoma premium edition (GMPE) software analysis—demonstrated normal perioptic retinal nerve fiber layer scans in the right eye, and, for the most part, similar findings in the left. Bruch’s membrane opening (BMO) measurements in the right were not suspicious, whereas those of the left demonstrated a thinned temporal neuroretinal rim in the area of the papillomacular bundle, and a relatively normal BMO in the superior temporal and inferior temporal sectors (Figure 1). The macular thickness scans were suspect in the left eye for early loss in the inferior arcuate region (Figure 2).
Discussion
In glaucoma, even in advanced cases, the remaining neuroretinal rim, though thin and excavated, is not usually pale and atrophic. In cases of non-glaucomatous optic neuropathies, there is pallor to the neuroretinal rim and rim thinning usually does not occur.
In our case, the patient has a bit of thinning of the neuroretinal rim in the left eye and pallor of the neuroretinal rim. With two diagnoses— normal tension glaucoma and a previous infarct affecting the optic nerve—two patient management plans must be created.
Let’s tackle the optic nerve infarct first. What we don’t have is a complete history of acute vision loss. But we do have three important findings, two of which are a visual field defect and optic nerve pallor. The field study in the left eye is consistent with a previous infarct, as non-glaucomatous optic neuropathies do present with a central or a cecocentral field defect. Following the initial infarct, the damaged tissue becomes pale and atrophic, and observation of the nerve clearly shows temporal pallor. The third, and probably most important, piece is the OCT image, which shows thinning of the temporal sector of the optic nerve, specifically in the area of the papillomacular bundle. Glaucomatous thinning usually occurs infero- and superotemporally, rather than in the papillomacular bundle. Given these findings, it does appear that the patient did, at some point, experience an optic nerve infarct.
The question as to whether or not the patient has normal tension glaucoma is not as clear cut. The thin appearance of the neuroretinal rim of the left eye from 1 to 5 is consistent with glaucomatous damage. While the visual field is suggestive of early glaucomatous damage, it is not diagnostic, as the questionable areas are contiguous with the central field defect (which is attributable to the ischemic event). But one cannot argue with the thin neuroretinal rim findings.
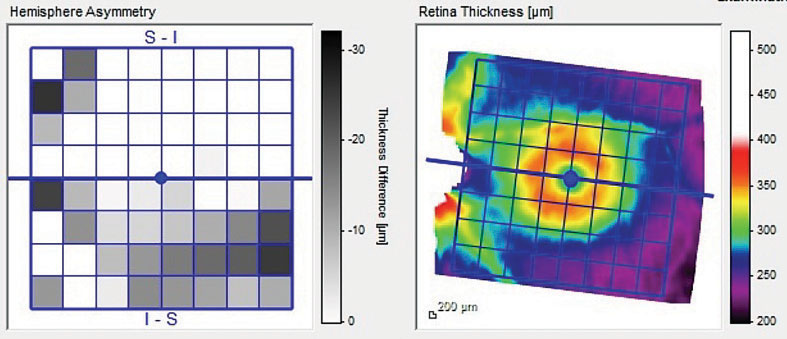 |
| Fig. 2. This image demonstrates the hemispheric asymmetry of the left macular scan between the hemisphere above and below the horizontal raphe. |
Management
So ultimately, the question moves to patient management. Once again, the infarction end of the diagnosis is straightforward: the damage has been done, and there’s nothing that needs to be done in this regard, other than continued monitoring. The glaucomatous end of the diagnosis is not as straightforward. But the question is that if the patient does in fact have normotensive glaucoma, how “at risk” is that left disc for progressive damage? And the answer to that specific question is simply, “no more than any other normotensive glaucoma patient.”
At this point, we have two valid options to manage this patient: intervene with medications or passively monitor the patient. But given that we’re not 100% certain the patient has glaucoma, as well as the absence of increased risk of more rapid progression than any other glaucoma patient, I chose to monitor the patient. I’ve only had the opportunity to see him twice over the course of a month. In other words, I don’t have longitudinal data to base a decision on, so I chose to monitor him, with the intention of watching him closely. If he does have normotensive glaucoma, changes will be visible to either the neuroretinal rim or the visual field, or both, over time, and he would then go on medication for glaucoma.

